Regeneron, Sanofi Get CHMP Backing for Expanded Libtayo Use
May 24 2021 - 6:18AM
Dow Jones News
By Colin Kellaher
Regeneron Pharmaceuticals Inc. and Sanofi SA Monday said the
European Medicines Agency's Committee for Medicinal Products for
Human Use recommended expanded approval of Libtayo as monotherapy
in a pair of advanced cancers.
The companies said the recommendations cover Libtayo for the
first-line treatment of adults with non-small cell lung cancer
expressing PD-L1 in >=50% of tumor cells with no EGFR, ALK or
ROS1 aberrations and for adults with locally advanced or metastatic
basal cell carcinoma who have progressed on or are intolerant to a
hedgehog pathway inhibitor.
Regeneron, of Tarrytown, N.Y., and France's Sanofi are jointly
developing and commercializing Libtayo under a global collaboration
agreement.
The European Commission, which generally follows the CHMP's
advice, is expected to make a decision on both indications in the
coming months, the companies said.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
May 24, 2021 06:13 ET (10:13 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
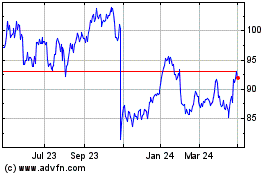
Sanofi (EU:SAN)
Historical Stock Chart
From Mar 2024 to Apr 2024
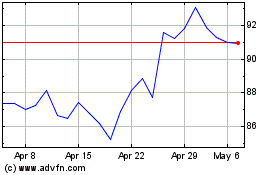
Sanofi (EU:SAN)
Historical Stock Chart
From Apr 2023 to Apr 2024