As filed with the Securities and Exchange Commission on December 9,
2019
Registration No.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF
1933
Premier Biomedical, Inc.
(Exact name of registrant as specified in its charter)
|
Nevada
|
2836
|
27-2635666
|
|
(State
or other jurisdiction of incorporation or organization
|
(Primary
Standard Industrial Classification Code Number)
|
(I.R.S.
Employer Identification No.)
|
|
P.O.
Box 25
Jackson
Center, PA 16133
|
(814)
786-8849
|
|
(Address,
including zip code, of registrant’s principal executive
offices)
|
(Telephone
number, including area code)
|
William
A. Hartman
Chief
Executive Officer
Premier
Biomedical, Inc.
P.O.
Box 25
Jackson
Center, PA 16133
(814)
786-8849
(Name,
address, including zip code, and telephone number, including
area code, of agent for service)
COPIES
TO:
Brian
A. Lebrecht, Esq.
Clyde
Snow & Sessions, PC
201 S.
Main Street, 13th Floor
Salt
Lake City, UT 84111
(801)
322-2516
Approximate
date of commencement of proposed sale to the public:
From
time to time after this registration statement becomes
effective.
If any
of the securities being registered on this Form are to be offered
on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, check the following box. [ X ]
If this
Form is filed to register additional securities for an offering
pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement
number of the earlier effective registration statement for the same
offering. [ ]
If this
Form is a post-effective amendment filed pursuant to Rule 462(c)
under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier
effective registration statement for the same offering. [
]
If this
Form is a post-effective amendment filed pursuant to Rule 462(d)
under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier
effective registration statement for the same offering. [
]
Indicate
by check mark whether the registrant is a large accelerated filer,
an accelerated filer, a non-accelerated filer, or a smaller
reporting company. See definitions of “large accelerated
filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-2 of the Exchange Act. (Check
one):
|
Large
accelerated filer
|
☐
|
Accelerated
filer
|
☐
|
|
Non-accelerated
filer
|
☐
|
Smaller
reporting company
|
☑
|
|
(Do not
check if a smaller reporting company)
|
|
Emerging growth
company
|
☐
|
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended
transition period for complying with any new or revised financial
accounting standards provided to Section 7(a)(2)(B) of the
Securities Act. [ ]
CALCULATION
OF REGISTRATION FEE
|
Title of Each
Class of Securities to be Registered
|
Amount to be
Registered (1)
|
Proposed Maximum
Offering Price Per Share (2)
|
Proposed Maximum
Aggregate Offering Price
|
Amount of
Registration Fee (3)
|
|
Shares of Common
Stock, par value $0.00001 per share
|
1,000,000,000
|
$0.0006
|
$600,000
|
$77.88
|
(1)
We are registering
1,000,000,000 shares of our common
stock that we will sell to Green Coast Capital International SA
pursuant to an Equity Purchase Agreement dated October 3, 2019,
which together shall have an aggregate initial offering price not
to exceed $5,000,000.
In the
event the maximum aggregate offering price is reached, any
remaining unsold shares shall be removed from
registration. The proposed maximum offering price per
share will be determined by the registrant in connection with the
issuance by the registrant of the securities registered
hereunder.
(2)
Estimated solely
for the purpose of computing the registration fee pursuant to Rule
457(c) of the Securities Act of 1933, as amended. Price per share
is based on the average of the high and low prices per share of our
common stock reported in the consolidated reporting system as
reported on the Pink Sheets Current Marketplace maintained by OTC
Markets, Inc. on December 3, 2019.
(3)
Calculated pursuant
to Rule 457(o) based on an estimate of the proposed maximum
aggregate offering price.
The
registrant hereby amends this registration statement on such date
or dates as may be necessary to delay its effective date until the
registrant shall file a further amendment which specifically states
that this registration statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933 or
until the registration statement shall become effective on such
date as the Commission, acting pursuant to said Section 8(a), may
determine.
The information in this Prospectus is not complete and may be
changed. We may not sell these securities until the registration
statement filed with the SEC is effective. This Prospectus is not
an offer to sell and it is not soliciting an offer to buy these
securities in any state where the offer or sale is not
permitted.
|
Preliminary Prospectus
|
Subject to Completion
|
Dated [•], 2019
|
PROSPECTUS
Up to
1,000,000,000 shares of common stock
We are
hereby registering 1,000,000,000 shares, representing 50% of our
authorized common stock1, for sale by Green
Coast Capital International SA, a Panama corporation and an
underwriter in this offering, pursuant to an Equity Purchase
Agreement. The agreement allows us to require Green Coast to
purchase up to $5,000,000 of our common stock.
We are
not selling any shares of common stock in the resale
offering. We, therefore, will not receive any proceeds
from the sale of the shares by the selling
shareholder. We will, however, receive proceeds from the
sale of securities to Green Coast pursuant to Put Notice(s) under
the Equity Purchase Agreement.
This
offering will terminate on the earlier of (i) when all
1,000,000,000 shares are sold, (ii) when the maximum offering
amount of $5,000,000 has been achieved, or (iii) on January
[•], 2022, unless we terminate it
earlier.
Investing
in the common stock involves risks. Premier Biomedical, Inc. has
limited operations, limited income, and limited assets, and you
should not invest unless you can afford to lose your entire
investment. See “Risk Factors” beginning on page 5.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of these
securities or determined if this Prospectus is truthful or
complete. Any representation to the contrary is a criminal offense.
Our common stock is governed under The Securities Enforcement and
Penny Stock Reform Act of 1990, and as a result you may be limited
in your ability to sell our stock.
Our
common stock is registered under Section 12(g) of the Securities
Exchange Act of 1934 and is quoted on the Pink Sheets Current
Marketplace maintained by OTC Markets, Inc. under the symbol
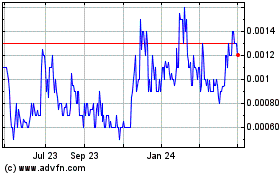
“BIEI.” The closing price of our common stock as
reported on the Pink Sheets Current on December 3, 2019 was
$0.0006.
These
shares will be sold by Green Coast from time to time whenever the
person or persons who exercise voting control over Green Coast deem
it appropriate and for whatever reason the person or persons
who exercise voting control over Green Coast deem it appropriate in
the over-the-counter market or other national securities exchange
or automated interdealer quotation system on which our common stock
is then listed or quoted, through negotiated transactions or
otherwise at market prices prevailing at the time of sale or at
negotiated prices. We provide more information about how the
Selling Shareholders may sell their shares of common stock in the
section of this prospectus entitled “Plan of Distribution” beginning
on page 25.
We will
bear all costs associated with this registration
statement.
Green
Coast, and any participating broker-dealers, will be deemed to be
“underwriters” within the meaning of the Securities Act
of 1933, as amended, or the “Securities Act,” and any
commissions or discounts given to any such broker-dealer may be
regarded as underwriting commissions or discounts under the
Securities Act. Green Coast will purchase the shares of our common
stock for ninety percent (90%) of the
lowest closing trade price of the common stock during the five (5)
trading days immediately following the date Green Coast receives
shares of our common stock pursuant to a put notice issued under
the Equity Purchase Agreement. Green Coast has informed us
that they do not have any agreement or understanding, directly or
indirectly, with any person to distribute their common
stock.
Neither
the Securities and Exchange Commission nor any state securities
commission has approved or disapproved of these securities or
determined if this Prospectus is truthful or complete. Any
representation to the contrary is a criminal offense. Our common
stock is governed under The Securities Enforcement and Penny Stock
Reform Act of 1990, and as a result you may be limited in your
ability to sell our stock.
The date of this Prospectus is [•], 2019.
Table of Contents
|
|
|
Page
|
|
Part
I
|
|
Prospectus
Summary
|
|
1
|
|
Risk
Factors
|
|
4
|
|
Use of
Proceeds
|
|
20
|
|
Determination
of Offering Price
|
|
|
|
Dilution
|
|
|
|
Selling
Security Holders
|
|
21
|
|
Plan of
Distribution
|
|
22
|
|
Description
of Securities to be Registered
|
|
24
|
|
Interests
of Named Experts and Counsel
|
|
25
|
|
Description
of Business
|
|
26
|
|
Description
of Property
|
|
35
|
|
Legal
Proceedings
|
|
35
|
|
Selected
Financial Data
|
|
36
|
|
Management’s
Discussion and Analysis
|
|
37
|
|
Changes
in and Disagreements with Accountants on Accounting and Financial
Disclosure
|
|
48
|
|
Directors,
Executive Officers, Promoters, and Control Persons
|
|
49
|
|
Executive
Compensation
|
|
52
|
|
Security
Ownership of Certain Beneficial Owners and Management
|
|
55
|
|
Certain
Relationships and Related Transactions
|
|
56
|
|
Disclosure
of Commission Position on Indemnification for Securities Act
Liabilities
|
|
58
|
|
Where
You Can Find More Information
|
|
59
|
|
Experts
|
|
60
|
|
Index
to Financial Statements
|
|
F-1
|
ABOUT THIS PROSPECTUS
This
prospectus is part of a registration statement that we filed on
behalf of the Selling Shareholders with the Securities and Exchange
Commission (the “Commission”) to permit the Selling
Shareholders to sell the shares described in this prospectus in one
or more transactions. The Selling Shareholders and the plan of
distribution of the shares being offered by them are described in
this prospectus under the headings “Selling Shareholders” and
“Plan of
Distribution.”
You
should rely only on the information that is contained in this
prospectus. We and the Selling Shareholders have not authorized
anyone to provide you with information that is in addition to or
different from that contained in this prospectus. If anyone
provides you with different or inconsistent information, you should
not rely on it.
The
shares of common stock offered by this prospectus are not being
offered in any jurisdiction where the offer or sale of such common
stock is not permitted. You should not assume that the information
contained in this prospectus is accurate as of any date other than
the date of this prospectus regardless of the date of delivery of
this prospectus or any sale of the common stock offered by this
prospectus. Our business, financial condition, liquidity, results
of operations and prospects may have changed since those dates. The
rules of the Commission may require us to update this prospectus in
the future.
PROSPECTUS SUMMARY
PREMIER BIOMEDICAL, INC.
This
summary highlights selected information contained in greater detail
elsewhere in this prospectus. This summary does not contain all the
information you should consider before investing in our common
stock. You should read the entire prospectus, including our
financial statements and related notes and the information set
forth under the heading “Risk Factors” and
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations” before investing in our
common stock. In this prospectus, the “Company,”
“we,” “us,” and “our” refer to
Premier Biomedical, Inc.
We were
strictly a research-based company that intended to discover cures
for PTSD, cancer and various other diseases. In order to fund
on-going research and development in these areas, we developed a
line of topical hemp oil pain relief products. We began selling
these pain relief products in January of 2017 with a single product
and currently have eight topical pain relief products.
Through
our continued development and expansion of proprietary drugs and
treatments, we have reorganized the company into six technology
centers: (1) extra-corporeal treatment of disease, (2) PTSD
treatment, (3) anti-breast cancer drugs, (4) hemp oil/CBD pain
relief products, (5) anti-aging treatments, and (6) chemical and
alcohol addiction treatment.
Pain Management Products
We have
developed and are now marketing all-natural, hemp-oil based
products that are pesticide and solvent free. These products
provide generalized, neuropathic and localized topical pain
relief.
We
offer alternatives to dangerous and addictive opioid pain killers,
which are currently the principal contributors to roughly 200 drug
overdose deaths per day in the United States. In the past year we
have rapidly expanded our product offerings, and we now offer eight
pain relief products that are leaders in the pain-relief
field:
1.
96-hour pain relief
patch with 50 mg of hemp oil extract, the highest level of pain
relief ingredient available in the industry;
2.
120 mg/ 10 ml
water-based roll-on applicator;
3.
150 mg/ 10 ml
oil-based roll-on applicator;
4.
150 mg/ 30 ml
oil-based pump spray applicator;
5.
150 mg/ 2 oz.
ointment;
6.
200 mg/10 ml
oil-based roll-on applicator;
7.
500 mg/ 30 ml
oil-based pump spray applicator; and
8.
500 mg/ 1 oz.
ointment.
We
believe that this eight-product array positions us favorably in the
topical pain relief marketplace. The topical pain relief market is
expected to grow rapidly in the next few years, due to the focus on
reduction of opioid pain medication use, and we intend to be a
major player in that expanding market.
Now
that we have completed the product design and development phase, we
are aggressively embarking on the product distribution and sales
phase by:
1.
Expanding our
online sales beyond our web site at:
www.painreliefmeds.com;
2.
Securing the
services of a social media coordinator to ensure that we optimize
that promotional tool;
3.
Recruiting a
National Sales Director to coordinate our growing field of sales
representatives and distributors;
4.
Securing the
services of a sales organization with expertise in marketing to the
government and senior care facilities;
5.
Engaging an
investor relations firm to facilitate television appearances
designed to gain optimum exposure for our company and its
products;
6.
Appearing in radio
and television broadcasts, and podcasts, via Uptick Newswire
periodically to ensure that our story gets out to the public;
and
7.
Retaining the
services of marketing firms to promote the Company and its products
through social media.
8.
Establishing
relationships with major distributors who will blanket specialized
sales outlets such as pharmacies, doctors’ offices,
convenience stores, long-term care facilities, large retail
facilities, etc.
In
addition, we are in the process of seeking potential partnerships
outside the United States to manufacture and market our products
worldwide. We anticipate that these partnerships will make new
markets available to us and allow us to rapidly increase our sales
and profitability through favorable manufacturing
arrangements.
Customers indicate
that they were able to achieve pain relief from our products and
stop the use of opioid painkillers. Public awareness of the harmful
side effects of opioid painkillers has grown significantly, and
many states have initiated litigation against drug makers claiming
they misrepresented the risks of opioid painkillers.2 As patients seek to
cut back their use of opioid painkillers and look for alternatives,
we believe demand for our products will see a significant increase.
We intend to petition national insurance agencies to urge them to
consider covering the use of our all-natural pain relief products
as a safe alternative to opioid painkillers.
Corporate Information
We
were incorporated on May 10, 2010 in the State of Nevada. We have
two wholly-owned subsidiaries, Premier Biomedical Pain Relief Meds,
LLC, a Nevada limited liability company organized on September 14,
2017, and Health Stations, LLC, a Nevada limited liability company
organized on August 28, 2019.
Our
corporate headquarters are located in Jackson Center, PA. Our
mailing address is P.O. Box 25, Jackson Center, PA 16133,
and our telephone number is (724) 633-7033. We have offices
virtually in the homes of our management team who
reside in Pennsylvania, Michigan and various other states. Our
websites are www.premierbiomedical.com and
www.painreliefmeds.com.
Information contained on our website is not incorporated into, and
does not constitute any part of, this Prospectus.
The Offering
We are
registering up to 1,000,000,000 shares of our common stock for
resale by Green Coast Capital International SA, a Panama
corporation and an underwriter in this offering, pursuant to an
Equity Purchase Agreement. The agreement allows us to require Green
Coast to purchase up to $5,000,000 of our common
stock.
These
shares will be sold by Green Coast from time to time whenever and
for whatever reason the person or persons who exercise voting
control over Green Coast deem it appropriate in the
over-the-counter market or other national securities exchange or
automated interdealer quotation system on which our common stock is
then listed or quoted, through negotiated transactions or otherwise
at market prices prevailing at the time of sale or at negotiated
prices.
Green
Coast will purchase the shares of our common stock for ninety
percent (90%) of the lowest closing
bid price of the common stock during the five consecutive trading
days immediately following following the Clearing Date
associated with our Put Notice. Green Coast received a convertible
promissory note in the principal amount of $150,000, for which they
paid $25,000 cash, as a commitment for the investment. The shares
of our common stock issuable upon conversion of the note are not
included in this registration statement. Green Coast has informed
us that they do not have any agreement or understanding, directly
or indirectly, with any person to distribute our common
stock.
We will
not be permitted to submit a Put Notice to Green Coast, or draw
down any funds from the financing arrangement, if the shares issued
to Green Coast would cause them to beneficially own more than 4.99%
of our outstanding common stock on the date of the issuance of the
shares. The 1,000,000,000 shares being registered represent a good
faith estimate of the number of shares of common stock that will be
issuable pursuant to the agreement.
On
any Closing Date, we shall deliver to Green Coast the number of
shares of the Common Stock registered in the name of Green Coast as
specified in the Put Notice. In addition, we must deliver the other
required documents, instruments and writings required. Green Coast
is not required to purchase the shares unless:
●
Our Registration
Statement with respect to the resale of the shares of Common Stock
delivered in connection with the applicable put shall have been
declared effective;
●
We shall have
obtained all material permits and qualifications required by any
applicable state for the offer and sale of the Registrable
Securities; and
●
We shall have filed
with the SEC in a timely manner all reports, notices and other
documents required.
Green
Coast has agreed that neither it nor its affiliates will engage in
any short selling of the common stock.
All of
the common stock registered by this Prospectus will be sold by
Green Coast at the prevailing market prices at the time they are
sold. We currently have 186,961,480 shares of common stock
outstanding, and if all of the shares included in the registration
statement of which this Prospectus is a part are issued, and all
outstanding warrants are exercised, we will have over 1.2 billion
shares of common stock outstanding.
RISK FACTORS
Any
investment in our common stock involves a high degree of risk. You
should consider carefully the following information, together with
the other information contained in this Annual Report, before you
decide to buy our common stock. If one or more of the following
events actually occurs, our business will suffer, and as a result
our financial condition or results of operations will be adversely
affected. In this case, the market price, if any, of our common
stock could decline, and you could lose all or part of your
investment in our common stock.
Currently, our
focus is on the development and distribution of our pain products.
We are also developing medical treatments for Alzheimer’s
disease, multiple sclerosis, amyotrophic lateral sclerosis,
fibromyalgia, traumatic brain injury, blood sepsis and viremia, and
cancer. We face risks in developing our product candidates and
services and eventually bringing them to market. We also face risks
that our business model may become obsolete. The following risks
are material risks that we face. If any of these risks occur, our
business, our ability to achieve revenues, our operating results
and our financial condition could be seriously harmed.
Risk Factors Related to the Offering
Existing stockholders may experience significant dilution from the
sale of our common stock pursuant to the Green Coast Equity
Purchase Agreement.
The
sale of our common stock to Green Coast Capital International SA in
accordance with the Equity Purchase Agreement may have a dilutive
impact on our shareholders. As a result, our net income per share
could decrease in future periods and the market price of our common
stock could decline. In addition, the lower our stock price is at
the time we exercise our put options, the more shares of our common
stock we will have to issue to Green Coast in order to exercise a
put under the Equity Purchase Agreement. If our stock price
decreases, then our existing shareholders would experience greater
dilution for any given dollar amount raised through the
Offering.
The
perceived risk of dilution may cause our stockholders to sell their
shares, which may cause a decline in the price of our common stock.
Moreover, the perceived risk of dilution and the resulting downward
pressure on our stock price could encourage investors to engage in
short sales of our common stock. By increasing the number of shares
offered for sale, material amounts of short selling could further
contribute to progressive price declines in our common
stock.
The issuance of shares pursuant to the Green Coast Equity Purchase
Agreement may have a significant dilutive effect.
Depending
on the number of shares we issue pursuant to the Green Coast Equity
Purchase Agreement, it could have a significant dilutive effect
upon our existing shareholders. Although the number of shares that
we may issue pursuant to the Equity Purchase Agreement will vary
based on our stock price (the higher our stock price, the less
shares we have to issue) the information set out below indicates
the potential dilutive effect to our shareholders, based on
different potential future stock prices, if the full amount of the
Equity Purchase Agreement is realized.
Dilution based upon common stock put to Green
Coast and the stock price discounted to Green Coast’s
purchase price of 90% of the lowest closing bid price of the common
stock during the five consecutive trading days immediately
following the Clearing Date associated with our Put
Notice. The example below illustrates
dilution based upon a $0.0006 market price/$0.00054 purchase price
and other increased/decreased prices (without regard to Green
Coast’s 4.99% ownership limit):
$5,000,000 Put
|
Stock Price (Green Coast Purchase Price)
|
|
Percentage of Outstanding Shares (1)
|
|
$0.00075
($0.000675) +25%
|
7.4 billion
|
97%
|
|
$0.0006
($0.00054)
|
9.3 billion
|
98%
|
|
$0.00045
($0.000405) -25%
|
12.3 billion
|
99%
|
|
(1)
|
Based on 200,000,000 shares outstanding before the first Put, as of
December 3, 2019.
|
Green Coast Capital Group, LLC will pay less than the
then-prevailing market price of our common stock which could cause
the price of our common stock to decline.
Our common stock to be issued to Green Coast under
the Equity Purchase Agreement will be purchased at a ten percent
(10%) discount or ninety percent (90%) of the lowest closing bid
price of the common stock during the five consecutive trading days
immediately following the Clearing Date associated with our
Put Notice.
Green
Coast has a financial incentive to sell our shares immediately upon
receiving the shares to realize the profit between the discounted
price and the market price. If Green Coast sells our shares, the
price of our common stock may decrease. If our stock price
decreases, Green Coast may have a further incentive to sell such
shares. Accordingly, the discounted sales price in the Equity
Purchase Agreements may cause the price of our common stock to
decline.
Green Coast Capital International SA has entered into similar
agreements with other public companies and may not have sufficient
capital to meet our put notices.
Green
Coast has entered into similar investment agreements with other
public companies, and some of those companies have filed
registration statements with the intent of registering shares to be
sold to Green Coast pursuant to investment agreements. We do not
know if management at any of the companies who have or will have
effective registration statements intend to raise funds now or in
the future, what the size or frequency of each put request would
be, if floors will be used to restrict the amount of shares sold,
or if the investment agreement will ultimately be cancelled or
expire before the entire amount of shares are put to Green Coast.
Since we do not have any control over the requests of these other
companies, if Green Coast receives significant requests, it may not
have the financial ability to meet our requests. If so, the amount
of available funds may be significantly less than we
anticipate.
We are registering an aggregate of 1,000,000,000 shares of common
stock to be issued under the Green Coast Equity Purchase Agreement.
The sale of such shares could depress the market price of our
common stock.
We
are registering an aggregate of 1,000,000,000 shares of common
stock under the registration statement of which this Prospectus
forms a part for issuance pursuant to the Green Coast Equity
Purchase Agreement. The sale of these shares into the public market
by Green Coast could depress the market price of our common
stock.
Risk Factors Related to the Business of the Company
We have a limited operating history and our financial results are
uncertain.
We have
a limited history and face many of the risks inherent to a new
business. As a result of our limited operating history, it is
difficult to accurately forecast our potential revenue. We were
incorporated in Nevada in 2010. Our revenue and income potential is
unproven and our business model is still emerging. Therefore,there
can be no assurance that we will provide a return on investment in
the future. An investor in our common stock must consider the
challenges, risks and uncertainties frequently encountered in the
establishment of new technologies, products and processes in
emerging markets and evolving industries. These challenges include
our ability to:
●
execute our
business model;
●
create brand
recognition;
●
manage growth in
our operations;
●
create a customer
base in a cost-effective manner;
●
access additional
capital when required; and
●
attract and retain
key personnel.
There
can be no assurance that our business model will be successful or
that it will successfully address these and other challenges, risks
and uncertainties.
We will need additional funding in the future, and if we are unable
to raise capital when needed, we may be forced to delay, reduce or
eliminate our product candidate development programs, commercial
efforts, or sales efforts.
Developing products
and methods and procedures of treatment and marketing developed
products is costly. We will need to raise substantial additional
capital in the future in order to execute our business plan and
help us and our collaboration partners fund the development and
commercialization of our product candidates.
In 2014
and through 2019, we raised funds through public and private equity
offerings. We may need to finance future cash needs through public
or private equity offerings, debt financings or strategic
collaboration and licensing arrangements. To the extent that we
raise additional funds by issuing equity securities, our
shareholders may experience additional dilution, and debt
financing, if available, may involve restrictive covenants and may
result in high interest expense. If we raise additional funds
through collaboration and licensing arrangements, it may be
necessary to relinquish some rights to our product candidates,
processes and technologies or our development projects or to grant
licenses on terms that are not favorable to us. We cannot be
certain that additional funding will be available on acceptable
terms, or at all. If adequate funds are not available from the
foregoing sources, we may consider additional strategic financing
options, including sales of assets, or we may be requiredto delay,
reduce the scope of, or eliminate one or more of our research or
development programs or curtail some of our commercialization
efforts of our operations. We may seek to access the public or
private equity markets whenever conditions are favorable, even if
we do not have an immediate need for additional
capital.
Negative public perception of hemp and cannabis-related businesses,
misconceptions about the nature of our business and regulatory
uncertainties could have a material adverse effect on our business,
financial condition, and results of operations.
The hemp plant
and the cannabis/marijuana plant are both part of the
same cannabis sativa
genus species of plant, except that hemp, by
definition, has less than 0.3% tetrahydrocannabinol
(“THC”) content and is legal under federal and state
laws, but the same plant with a higher THC content is
cannabis/marijuana, which is legal under certain state laws, but
which is not legal under federal law. The similarities between
these plants can cause confusion, and our activities with
legal hemp may be incorrectly perceived as us being
involved in federally illegal cannabis/marijuana. Also, despite
growing support for the cannabis/marijuana industry and
legalization of cannabis/marijuana in certain U.S. states, many
individuals and businesses remain opposed to the cannabis/marijuana
industry. Any negative press resulting from any incorrect
perception that we have entered into the cannabis/marijuana space
could result in a loss of current or future business. It could also
adversely affect the public’s perception of us and lead to
reluctance by new parties to do business with us or to own our
common stock.
Certain
retailers, like Amazon, do not allow the sale of products
containing CBD. Other platforms such as Facebook and Google have
policies that restrict advertising of CBD products. Until
regulators provide more definitive and consistent rules for CBD
products, many retailers, distributors and business partners tend
to avoid getting involved in CBD businesses because of the
uncertainty of what regulators may do. Misunderstandings about the
legal nature of our business and the difference between CBD and
marijuana may also discourage some business partners and customers
from working with us or purchasing our products.
We
cannot assure you that additional business partners, including but
not limited to online retailers, distributors, financial
institutions and customers, will not attempt to end or curtail
their relationships with us. Any such negative press or cessation
of business could have a material adverse effect on our business,
financial condition, and results of operations.
U.S. federal, state and foreign regulation and enforcement of laws
relating to cannabis and its derivatives may adversely affect our
ability to sell our products and our revenue.
There
are (i) thirty-three (33) states in the United States, the District
of Columbia, Guam and Puerto Rico have approved comprehensive
public medical marijuana/cannabis programs. Approved Efforts in
another thirteen (13) states allow use of low THC, high CBD
products for medical reasons in limited situations or as a legal
defense. Ten (10) of these states and the District of Columbia have
legalized cannabis/marijuana for adult recreational
use. This leaves only
four states (Idaho, Kansas, Conversely, under the federal
Controlled Substances Act (the “CSA”), the policies and
regulations of the federal government and its agencies are that
cannabis/marijuana has no medical benefit and a range of activities
are prohibited, including cultivation, possession, personal use,
and interstate distribution of cannabis/marijuana. In the event the
U.S. Department of Justice (the “DOJ”) begins strict
enforcement of the CSA in states that have laws legalizing medical
and/or adult recreational cannabis/marijuana, there may be a direct
and adverse impact to any future business or prospects that we may
have in the cannabis/marijuana business. Even in those
jurisdictions in which the manufacture and useof medical
cannabis/marijuana has been legalized at the state level, the
possession, use, and cultivation of cannabis/marijuana all remain
violations of federal law that are punishable by imprisonment and
substantial fines. Moreover, individuals and entities may violate
federal law if they intentionally aid and abet another in violating
these federal controlled substance laws, or conspire with another
to violate them.
For
example, the California Bureau of Cannabis Control sent nine
hundred (900) warning letters to marijuana shops suspected of
operating without a state license. The Bureau also issued a
cease-and-desist letter to the operator of an online directory of
marijuana dispensaries, products, and delivery services. The letter
threatened fines and criminal penalties if the company did not
remove the listings for unlicensed marijuana businesses. Likewise,
if we unknowingly do business with unlicensed entities or list them
on our website, we may be subject to similar regulatory action that
would halt our operations and affect our financial
performance.
Local,
state, federal, and international hemp and
cannabis/marijuana laws and regulations are broad in scope and
subject to evolving interpretations, which could require us to
incur substantial costs associated with compliance requirements. In
addition, violations of these laws, or allegations of such
violations, could disrupt our business and result in a material
adverse effect on our operations. In addition, it is possible that
cannabinoid-related regulations may be enacted in the future that
will be directly applicable to our business. It is also possible
that the federal government will begin strictly enforcing existing
laws, which may limit the legal uses of the hemp plant and its
derivatives and extracts, such as cannabinoids. However, our work
in hemp would continue since hemp research, development, and
commercialization activities are permitted under applicable federal
and state laws, rules, and regulations. Until Congress amends the
CSA or the executive branch deschedules or reschedules cannabis
under it, there is a risk that federal authorities may enforce
current federal law. Enforcement of the CSA by federal authorities
could impair the Company’s revenue and profit, and it could
even force the Company to cease manufacturing its products. The
risk of strict federal enforcement of the CSA in light of
congressional activity, judicial holdings, and stated federal
policy, including enforcement priorities, remains
uncertain.
Until
such time as the federal government reclassifies marijuana from a
Schedule 1 narcotic, we do not intend to pursue any
involvement in the marijuana business. At this time, we intend to
continue only in the federally legal hemp product business. When
Congress approved the 2018 Farm Bill, it defined hemp as an
agricultural product and differentiated it from marijuana. This
means hemp is not a controlled substance, and may be more broadly
cultivated. Hemp-derived products may now be transferred across
state lines for commercial purposes. The new law also allows for
the sale, transport, or possession of hemp-derived products, so
long as those items are produced in a manner consistent with the
law. There are several restrictions that apply to those who
cultivate hemp and produce hemp-derived products. Key among these
restrictions is that hemp cannot contain more than 0.3 percent
THC.
While
the 2018 Farm Bill legalized the cultivation of hemp and removed
hemp-derived substances from Schedule 1 of the CSA, it does not
legalize CBD generally. The FDA and DOJ continue to exercise
control over CBD and there is still some lack of clarity as to
exactly how CBD will be regulated going forward.
CBD has
been deemed relatively safe and, from now on, should not be subject
to international illicit drug scheduling according to a World
Health Organization (“WHO”) comprehensive review
published in July 2018. The WHO has formally submitted its
conclusion to United Nations Secretary-General António
Guterres, a prelude to this officially becoming the
case.
On June
25, 2018, the U.S. Food and Drug Administration (“FDA”)
approved CBD-based Epidiolex to treat severe forms of epilepsy.
This marked the groundbreaking admission by the FDA that cannabis
has medical value. On October 1, 2018, the DOJ placed
“FDA-approved drugs that contain CBD derived from cannabis
and no more than 0.1 percent THC” to Schedule 5 of the CSA.
This action is narrowly tailored to reschedule Epidiolex off of
Schedule 1 because the DOJ’s ability to remove all
restrictions from cannabis extracts, including CDB, is restricted
by the Single Convention on Narcotic Drugs, 1961.
Our product candidates are not approved by the FDA or other
regulatory authority, and we face risks of unforeseen medical
problems, and up to a complete ban on the sale of our product
candidates.
The
efficacy and safety of pharmaceutical products is established
through a process of clinical testing under FDA oversight. Our
products have not gone through this process because we believe that
the topical products we sell are not subject to this process.
However, if an individual were to use one of our products in an
improper manner, we cannot predict the potential medical harm to
that individual. If such an event were to occur, the FDA or similar
regulatory agency might impose a complete ban on the sale or use of
our products.
The FDA might not approve
our product candidates for marketing and sale.
We
intend to enter into agreements with larger pharmaceutical
companies as collaboration partners, in part to help cover the cost
of seeking regulatory approvals for our pharmaceutical and medical
product candidates. We believe that FDA approval of some of our
product candidates will need to undergo a full investigational new
drug (IND) application with the FDA, including clinical trials.
There can be no assurance that the FDA will approve our IND
application or any other applications. Failure to obtain the
necessary FDA approval will have a material negative affect on our
operations. While we intend to license our Feldetrex®
product to a larger pharmaceutical company, they in turn, may not
be able to obtain the necessary approval to market and sale the
product.
New regulations governing the introduction, marketing and sale of
our products to consumers could harm our business.
Our
pain management products have not been approved by the FDA or any
other regulatory agency, and the FDA does not have a
pre-market approval system for our pain management
products. However, our operations could be harmed if new laws or
regulations are enacted that restrict our ability to market or
distribute our products or impose additional burdens or
requirements on us in order to continue selling our products. In
addition, the adoption of new regulations or changes in the
interpretations of existing regulations may result in significant
compliance costs or discontinuation of product sales and may impair
the marketability of our products, resulting in significant loss of
net sales.
We have
observed a general increase in regulatory activity and activism in
the United States and the regulatory landscape is becoming more
complex with increasingly strict requirements. If this trend
continues, we may find it necessary to alter some of the ways we
have traditionally marketed our products in order to stay in
compliance with a changing regulatory landscape and this could add
to the costs of our operations and/or have an adverse impact on our
business.
We
cannot predict the nature of any future laws, regulations,
interpretations, or applications, nor can we determine what effect
additional governmental regulations or administrative orders, when
and if promulgated, would have on our business. Future changes
could include requirements to make certain changes to our products
to meet new standards, the recall or discontinuation of certain
products that cannot be changed, additional record keeping,
expanded documentation of the properties of certain products,
expanded or different labeling, and additional scientific
substantiation. Any or all of these requirements could have a
material adverse effect on our business, financial condition, and
operating results.
We may fail to deliver commercially successful new product
candidates, methods and procedures of treatment, and
treatments.
Our
technology is at an early stage of research and development. We are
also actively engaged in research and development of new
products.
The
development of commercially viable new products and methods and
procedures of treatment, as well as the development of additional
uses for existing products and methods and procedures of treatment,
is critical to our ability to generate sales and/or sell the rights
to manufacture and distribute our product and process candidates to
another firm. Developing new products and methods and procedures of
treatment is a costly, lengthy and uncertain process. A new product
or process candidate can fail at any stage of the development or
commercialization, and one or more late-stage product or process
candidates could fail to receive regulatory approval.
New
product and process candidates may appear promising in development,
but after significant investment, fail to reach the market or have
only limited commercial success. This, for example, could be as a
result of efficacy or safety concerns, inability to obtain
necessary regulatory approvals, difficulty or excessive costs to
manufacture, erosion of patent term as a result of a lengthy
development period, infringement of third-party patents or other
intellectual property rights of others or inability to
differentiate the product or process adequately from those with
which it competes.
The commercialization of product and process candidates under
development may not be profitable.
In
order for the commercialization of our product candidates to be
profitable, our product and process candidates must be
cost-effective and economical to manufacture on a commercial scale.
Furthermore, if our product candidates and methods and procedures
of treatment do not achieve market acceptance, we may not be
profitable. Subject to regulatory approval, we expect to incur
significant development, sales and marketing expenses in connection
with the commercialization of our new product and process
candidates. Even if we receive additional financing, we may not be
able to complete planned development and marketing of any or all of
our product or process candidates. Our future profitability may
depend on many factors, including, but not limited to:
●
the terms and
timing of any collaborative, licensing and other arrangements that
we may establish;
●
the costs of
filing, prosecuting, defending and enforcing any patent claims and
other intellectual property rights;
●
the costs of
establishing manufacturing and production, sales, marketing and
distribution capabilities; and
●
the effect of
competing technological and market developments.
Even if
our collaboration partners receive regulatory approval for our
product and process candidates, we may not earn significant
revenues from such product or process candidates. With respect to
the product and methods and procedures of treatment candidates in
our development pipeline that are being developed by or in close
conjunction with third parties, our ability to generate revenues
from such product and process candidates will depend in large part
on the efforts of such third parties. To the extent that our
collaboration partners are not successful in commercializing our
product or process candidates, our revenues will suffer, we will
incur significant additional losses and the price of our common
stock will be negatively affected.
We may engage in strategic transactions that fail to enhance
shareholder value.
From
time to time, we may consider possible strategic transactions,
including the potential acquisitions or licensing of products or
technologies or acquisition of companies, and other alternatives
with the goal of maximizing shareholder value. We may never
complete a strategic transaction, and in the event that we do
complete a strategic transaction, implementation of such
transactions may impair shareholder value or otherwise adversely
affect our business. Any such transaction may require us to incur
non-recurring or other charges and may pose significant integration
challenges and/or management and business disruptions, any of which
could harm our results of operation and business
prospects.
Our business is heavily regulated by governmental authorities, and
failure to comply with such regulation or changes in such
regulations could negatively impact our financial
results.
We must
comply with a broad range of regulatory controls on the testing,
approval, manufacturing and marketing of our product candidates,
procedures and other treatments, particularly in the United States
and countries of the European Union, that affect not only the cost
of product development but also the time required to reach the
market and the uncertainty of successfully doing so. Health
authorities have increased their focus on safety when assessing the
benefit risk/balance of drugs in the context of not only initial
product approval but also in the context of approval of additional
indications and review of information regarding marketed products.
Stricter regulatory controls also heighten the risk of changes in
product profile or withdrawal by regulators on the basis of
post-approval concerns over product safety, which could reduce
revenues and can result in product recalls and product liability
lawsuits. There is also greater regulatory scrutiny, especially in
the United States, on advertising and promotion and in particular
on direct-to-consumer advertising.
The
regulatory process is uncertain, can take many years, and requires
the expenditure of substantial resources. In particular, proposed
human pharmaceutical therapeutic product requirements set by the
FDA in the United States, and similar health authorities in other
countries, require substantial time and resources to satisfy. We
may never obtain regulatory approval for our product and process
candidates.
We may not be able to gain or sustain market acceptance for our
services and product candidates.
Failure
to establish a brand and presence in the marketplace on a timely
basis could adversely affect our financial condition and results of
operations. Moreover, there can be no assurance that we will
successfully complete our development and introduction of new
products or product enhancements, or methods and procedures of
treatment or that any such product candidates or methods and
procedures of treatment will achieve acceptance in the marketplace.
We may also fail to develop and deploy new products and product
enhancements on a timely basis.
The market for pain management products is highly competitive, and
we may not be able to compete successfully.
We
intend to operate in highly competitive markets. We will likely
face competition both from proprietary products of large
international manufacturers and producers of generic pain
management products. Most of the competitors in the industry have
longer operating histories and significantly greater financial,
technical, marketing and other resources than us, and may be able
to respond more quickly than we can to new or changing
opportunities and customer requirements. Also, many competitors
have greater name recognition and more extensive customer bases
that they can leverage to gain market share. Such competitors are
able to undertake more extensive promotional activities, adopt more
aggressive pricing policies and offer more attractive terms to
purchasers than we can.
Significant product
innovations, technical advances or the intensification of price
competition by competitors could adversely affect our operating
results. We cannot predict the timing or impact of competitive
products or their potential impact on sales of our products under
development.
If any
of our major pain management products were to become subject to a
problem such as unplanned loss of patent protection, unexpected
side effects, regulatory proceedings, publicity affecting doctor or
consumer confidence or pressure from competitive products, or if a
new, more effective alternative should be introduced, the adverse
impact on our revenues and operating results could be
significant.
The market for products, methods and procedures of treatment and
services in the pharmaceuticals industry is highly competitive, and
we may not be able to compete successfully.
We
intend to operate in highly competitive markets. We will likely
face competition both from proprietary products of large
international manufacturers and producers of generic
pharmaceuticals. Most of the competitors in the industry have
longer operating histories and significantly greater financial,
technical, marketing and other resources than us, and may be able
to respond more quickly than we can to new or changingopportunities
and customer requirements. Also, many competitors have greater name
recognition and more extensive customer bases that they can
leverage to gain market share. Such competitors are able to
undertake more extensive promotional activities, adopt more
aggressive pricing policies and offer more attractive terms to
purchasers than we can.
Significant product
innovations, technical advances or the intensification of price
competition by competitors could adversely affect our operating
results. We cannot predict the timing or impact of competitive
products or their potential impact on sales of our product
candidates.
If any
of our major product candidates or methods and procedures of
treatment were to become subject to a problem such as unplanned
loss of patent protection, unexpected side effects, regulatory
proceedings, publicity affecting doctor or patient confidence or
pressure from competitive products and methods and procedures of
treatment, or if a new, more effective treatment should be
introduced, the adverse impact on our revenues and operating
results could be significant.
We are dependent on the services of key personnel and failure to
attract qualified management could limit our growth and negatively
impact our results of operations.
We are
highly dependent on the principal members of our management and
scientific staff and certain key consultants, including our Chief
Executive Officer and the Chairman of our Board of Directors. We
will continue to depend on operations management personnel with
pharmaceutical and scientific industry experience. At this time, we
do not know of the availability of such experienced management
personnel or how much it may cost to attract and retain such
personnel. The loss of the services of any member of senior
management or the inability to hire experienced operations
management personnel could have a material adverse effect on our
financial condition and results of operations.
If physicians and patients do not accept our current or future
product candidates or methods and procedures of treatment, we may
be unable to generate significant additional revenue, if
any.
The
products and methods and procedures of treatment that we may
develop or acquire in the future may fail to gain market acceptance
among physicians, health care payors, patients and the medical
community. Physicians may elect not to recommend these treatments
for a variety of reasons, including:
●
timing of market
introduction of competitive drugs;
●
lower demonstrated
clinical safety and efficacy compared to other drugs or
treatments;
●
lack of
cost-effectiveness;
●
lack of
availability of reimbursement from managed care plans and other
third-party payors;
●
lack of convenience
or ease of administration;
●
prevalence and
severity of adverse side effects;
●
other potential
advantages of alternative treatment methods; and
●
ineffective
marketing and distribution support.
If our
product candidates and processes fail to achieve market acceptance,
we would not be able to generate significant revenue.
We are exposed to the risk of liability claims, for which we may
not have adequate insurance.
Since
we participate in the CBD, pain management and pharmaceutical
industries, we may be subject to liability claims by employees,
customers, end users and third parties. We do not currently have
product liability insurance. We intend to have proper insurance in
place; however, there can be no assurance that any liability
insurance we purchase will be adequate to cover claims asserted
against us or that we will be able to maintain such insurance in
the future. We intend to adopt prudent risk management programs to
reduce these risks and potential liabilities; however, we have not
taken any steps to create these programs and have no estimate as to
the cost or time required to do so and there can be no assurance
that such programs, if and when adopted, will fully protect us. We
may not be able to put risk management programs in place, or obtain
insurance, if we are unable to retain the necessary expertise
and/or are unsuccessful in raising necessary capital in the future.
Adverse rulings in any legal matters, proceedings and other matters
could have a material adverse effect on our business.
Pre-clinical and
clinical trials are conducted during the development of potential
products and other treatments to determine their safety and
efficacy for use by humans. Notwithstanding these efforts, when our
treatments are introduced into the marketplace, unanticipated side
effects may become evident. Manufacturing, marketing, selling and
testing our product candidates under development or to be acquired
or licensed, entails a risk of product liability claims. We could
be subject to product liability claims in the event that our
product candidates, processes, or products under development fail
to perform as intended. Even unsuccessful claims could result in
the expenditure of funds in litigation and the diversion of
management time and resources, and could damage our reputation and
impair the marketability of our product candidates and processes.
While we plan to maintain liability insurance for product liability
claims, we may not be able to obtain or maintain such insurance at
a commercially reasonable cost. If a successful claim were made
against us, and we don’t have insurance or the amount of
insurance was inadequate to cover the costs of defending against or
paying such a claim or the damages payable by us, we would
experience a material adverse effect on our business, financial
condition and results of operations.
Other companies may claim that we have infringed upon their
intellectual property or proprietary rights.
We do
not believe that our product candidates and methods and procedures
violate third-party intellectual property rights; however, we have
not had an independent party conduct a study of possible patent
infringements. Nevertheless, we cannot guarantee that claims
relating to violation of such rights will not be asserted by third
parties. If any of our product candidates or methods and procedures
of treatment are found to violate third-party intellectual property
rights, we may be required to expend significant funds to
re-engineer or cause to be re-engineered one or more of those
product candidates or methods and procedures of treatment to avoid
infringement, or seek to obtain licenses from third parties to
continue offering our product candidates or methods and procedures
of treatment without substantial re-engineering, and such efforts
may not be successful.
In
addition, future patents may be issued to third parties upon which
our product candidates and methods and procedures of treatment may
infringe. We may incur substantial costs in defending against
claims under any such patents. Furthermore, parties making such
claims may be able to obtain injunctive or other equitable relief,
which effectively could block our ability to further develop or
commercialize some or all of our products or methods and procedures
of treatment in the United States or abroad, and could result in
the award of substantial damages against us. In the event of a
claim of infringement, we may be required to obtain one or more
licenses from third parties. There can be no assurance that we will
be able to obtain such licenses at a reasonable cost, if at all.
Defense of any lawsuit or failure to obtain any such license could
be costly and have a material adverse effect on our
business.
Our success depends on our ability to protect our proprietary
technology.
Our
success depends, to a significant degree, upon the protection of
our proprietary technology, and that of any licensors. Legal fees
and other expenses necessary to obtain and maintain appropriate
patent protection could be material. Insufficient funding may
inhibit our ability to obtain and maintain such protection.
Additionally, if we must resort to legal proceedings to enforce our
intellectual property rights, the proceedings could be burdensome
and expensive, and could involve a high degree of risk to our
proprietary rights if we are unsuccessful in, or cannot afford to
pursue, such proceedings.
Our
licensors have been granted three U.S. patents: Sequential
Extracorporeal Treatment of Bodily Fluids, U.S. Patent No.
9,216,386; Utilization of Stents for the Treatment of Blood Borne
Carcinomas, U.S. Patent No. 8,758,287; and Medication and
Treatmentfor Disease, U.S. Patent No. 8,865,733, in the areas of
cancer, sepsis, and multiple sclerosis. We expect these patents to
cover the medical treatments for multiple sclerosis, blood sepsis,
and cancer and be effective until 2029. Our licensors have licensed
these technologies to us pursuant to the terms of the license
agreements. We anticipate that other technologies that derive from
these patents will also belong to us and are covered by the license
agreements. However, we have not conducted thorough prior art or
novelty studies, but we are not aware of existing prior art that
would prevent us from obtaining patents on our product candidates
or methods and procedures of treatment. Prior art preventing us
from obtaining broad patent protection is a possibility. Inability
to obtain valid and enforceable patent protection would have a
material negative impact on our business opportunities and success.
Because the patent positions of pharmaceutical and biotechnology
companies are highly uncertain and involve complex legal and
factual questions, the patents may not be granted on our
applications, and any future patents owned and licensed by us may
not prevent other companies from developing competing products or
ensure that others will not be issued patents that may prevent the
sale of our products or require licensing and the payment of
significant fees or royalties. Furthermore, to the extent that:
(i) any of our future products or methods are not patentable;
(ii) such products or methods infringe upon the patents of
third parties; or (iii) our patents or future patents fail to
give us an exclusive position in the subject matter to which such
patents relate, our business will be adversely affected. We may be
unable to avoid infringement of third-party patents and may have to
obtain a license, or defend an infringement action and challenge
the validity of such patents in court. A license may be unavailable
on terms and conditions acceptable to us, if at all. Patent
litigation is costly and time consuming, and we may be unable to
prevail in any such patent litigation or devote sufficient
resources to even pursue such litigation. If we do not obtain a
license under such patents, are found liable for infringement and
are not able to have such patents declared invalid, we may be
liable for significant monetary damages, encounter significant
delays in bringing products to market or may be precluded from
participating in the manufacture, use or sale of products or
methods of treatment requiring such licenses.
We may
also rely on trademarks, trade secrets and contract law to protect
certain of our proprietary technology. There can be no assurance
that any trademarks will be approved, that such contract will not
be breached, or that if breached, we will have adequate remedies.
Furthermore, there can be no assurance that any of our trade
secrets will not become known or independently discovered by third
parties.
Additionally, we
may, from time to time, support and collaborate in research
conducted by universities and governmental research organizations.
There can be no assurance that we will have or be able to acquire
title or exclusive rights to the inventions or technical
information derived from such collaborations, or that disputes will
not arise with respect to rights in derivative or related research
programs conducted by us or such collaborators.
Our future growth may be inhibited by the failure to implement new
technologies.
Our
future growth is partially tied to our ability to improve our
knowledge and implementation of medical and pharmaceutical
technologies. The inability to successfully implement commercially
viable medical and pharmaceutical technologies in response to
market conditions in a manner that is responsive to our
customers’ requirements could have a material adverse effect
on our business.
We do not own certain of our technologies, they are owned by, and
licensed from, entities that are under the control of the Chairman
of our Board of Directors.
We do
not currently own the certain technologies necessary to conduct our
operations. The patents necessary to pursue our intended business
plan are under the control of our Chairman of the Board of
Directors. As consideration for the two licenses, we agreed to (i)
pay a royalty of five percent (5%) of any sales of products using
the technology, with no minimum royalty and (ii) reimburse the
licensor for any costs incurred in pursuing its proprietary rights
in the licensed technology and pay any costs incurred for
maintaining or obtaining the licensors’ proprietary rights in
the licensed technology in the U.S. and in extending the
intellectual property to other countries around the world. The
licensor has the sole discretion to select other countries into
which exclusive rights in the licensed technology may be pursued,
and if we decline to pay those expenses, then the licensor may pay
said expenses and our licensed rights in those countries will
revert to the licensor. The license agreements contain provisions
that require us to indemnify thelicensor for any claims, including
costs of litigation, brought against them related to the licenses,
and require us to maintain insurance that may be burdensome. In the
event of a breach of our obligations under the license agreements,
the licensors are entitled to various damages and remedies, up to
and including termination of said license agreements. The licensors
are entities under the control of Dr. Mitchell S. Felder, the
Chairman of our Board of Directors. While Dr. Felder is one of our
Company’s founders and the Chairman of our Board of
Directors, there can be no assurance that he will extend the offer
to license these technologies to us in the future as currently
contemplated.
We do not intend to take our Feldetrex® product candidate past
the development stage, but instead intend to enter into
collaboration agreements with collaboration partners. If we are
unable to enter into an agreement with collaboration partners, our
Feldetrex® product candidate cannot be marketed, and it will
not generate revenue for us.
We do
not intend to conduct clinical trials on our Feldetrex®
product candidate. We instead intend to enter into one or more
collaboration agreements with third parties to do so. However, we
have not entered into any such agreements, or discussions for any
such agreements, and we cannot guarantee that we will be successful
in doing so. If we do not find a collaboration partner, the
Feldetrex®
product candidate cannot be marketed, and it will not generate any
revenue for us.
The
failure to generate revenue from our Feldetrex®
product candidate will have a materially adverse effect on our
overall revenues, profitability.
Risks Related To Our Common stock
The market price of our common stock may be volatile and may be
affected by market conditions beyond our control.
The
market price of our common stock is subject to significant
fluctuations in response to, among other factors:
●
variations in our
operating results and market conditions specific to Biomedical
Industry companies;
●
changes in
financial estimates or recommendations by securities
analysts;
●
announcements of
innovations or new products or services by us or our
competitors;
●
the emergence of
new competitors;
●
operating and
market price performance of other companies that investors deem
comparable;
●
changes in our
board or management;
●
sales or purchases
of our common stock by insiders;
●
commencement of, or
involvement in, litigation;
●
changes in
governmental regulations; and
●
general economic
conditions and slow or negative growth of related
markets.
In
addition, if the market for stocks in our industry or the stock
market in general, experiences a loss of investor confidence, the
market price of our common stock could decline for reasons
unrelated to our business, financial condition or results of
operations. If any of the foregoing occurs, it could cause the
price of our common stock to fall and may expose us to lawsuits
that, even if unsuccessful, could be costly to defend and a
distraction to the board of directors and management.
If we default on our convertible notes and are unable to repay the
notes, we will not have the funds we need to operate our business
and may lose access to additional financing.
We are
currently in default on the Note issued on August 8, 2017 because
the Maturity Date has passed. Per the terms of the Notes, the
Selling Shareholders have the option to demand payment of 130% of
the outstanding principal amount of a Note and any accrued and
unpaid interest thereon. We are currently unable to pay these
amounts in full. If the Selling Shareholders elect to exercise this
right rather than convert the Notes, we could possibly face
litigation. If we repay the Notes or any part thereof, we may not
be able to satisfy the obligations we have to other business
partners and may be forced to cease our business operations. Any
action by the Selling Shareholders would adversely affect our
financial position and ability to operate.
If we are unable to pay the costs associated with being a public,
reporting company, we may be forced to discontinue
operations.
We
expect to have significant costs associated with being a public,
reporting company, which may raise substantial doubt about our
ability to continue as a going concern. Our ability to continue as
a going concern will depend on positive cash flow, if any, from
future operations and on our ability to raise additional funds
through equity or debt financing. If we are unable to achieve the
necessary product sales or raise or obtain needed funding to cover
the costs of operating as a public, reporting company, we may be
forced to discontinue operations.
If we do not continue to meet the eligibility requirements of the
Pink Sheets Current tier, our common stock may be removed from Pink
Sheets Current and moved for quotation on a lower tier of the
marketplace maintained by OTC Markets Group, Inc., which may make
it more difficult for investors to resell their shares due to
suitability requirements.
Our
common stock is currently quoted on the Pink Sheets Current tier of
the marketplace maintained by OTC Markets Group, Inc. The Pink
Sheets Current tier does not require a minimum bid price. If we are
removed from the Pink Sheets Current tier, our stock will be quoted
on a lower tier. Broker-dealers often decline to trade in
over-the-counter stocks that are quoted on the OTC Pink tier, or a
lower tier, given the market for such securities are often limited,
the stocks are more volatile, and the risk to investors is greater.
These factors may reduce the potential market for our common stock
by reducing the number of potential investors. This may make it
more difficult for investors in our common stock to sell shares to
third parties or to otherwise dispose of their shares. This could
cause our stock price to decline.
If we
move down from the OTC Pink Current tier, we may be unable to
restore eligibility for quotation of our common stock on the Pink
Sheets Current tier or the OTCQB tier, and this will have a
negative impact on our market price. The lower tiers maintained by
OTC Markets, Inc. does not provide as much liquidity as the Pink
Sheets Current tier or the OTCQB tier. Many broker-dealers will not
trade or recommend OTC Pink stocks for their
clients.
Our principal shareholders have the ability to exert significant
control in matters requiring shareholder approval and could delay,
deter, or prevent a change in control of our company.
William
A. Hartman and Dr. Mitchell S. Felder collectively own 157,031
shares of our outstanding common stock, 2,000,000 shares of our
Series A Convertible Preferred Stock (which is convertible into an
aggregate of 2,000,000 shares of our common stock), and through the
exercise of warrants could acquire another 1,782,040 shares of our
common stock. The shares of our preferred stock have 100 votes per
share, giving these two shareholders approximately 51% of our
current voting securities. As a result, they have the ability to
influence matters affecting our shareholders, including the
election of our directors, the acquisition or disposition of our
assets, and the future issuance of our shares. Because they control
such shares, investors may find it difficult to replace our
management if they disagree with the way our business is being
operated. Because the influence by these shareholders could result
in management making decisions that are in the best interest of
those shareholders and not in the best interest of the investors,
you may lose some or all of the value of your investment in our
common stock. Investors who purchase our common stock should be
willing to entrust all aspects of operational control to our
current management team.
We do not intend to pay dividends in the foreseeable
future.
We do
not intend to pay any dividends in the foreseeable future. We do
not plan on making any cash distributions in the manner of a
dividend or otherwise. Our Board presently intends to follow a
policy of retaining earnings, if any.
We have the right to issue additional common stock and preferred
stock without consent of shareholders. This would have the effect
of diluting investors’ ownership and could decrease the value
of their investment.
Following an
amendment to our articles of incorporation, which has already been
approved by our shareholders and is anticipated to take effect on
or about December 19, 2019, we will be authorized to issue up to
2,000,000,000 shares of common stock, of which there were
186,961,480 shares issued and outstanding as of November 12, 2019.
An additional 3,570,600 shares may be issued and outstanding if all
of our currently outstanding preferred stock and warrants were
exercised and converted into common stock. Our outstanding
convertible notes require a reserve of approximately 220
million shares.
In
addition, our certificate of incorporation authorizes the issuance
of shares of preferred stock, the rights, preferences, designations
and limitations of which may be set by the Board of Directors. Our
certificate of incorporation has authorized the issuance of up to
10,000,000 shares of preferred stock in the discretion of our
Board. The shares of authorized but undesignated preferred stock
may be issued upon filing of an amended certificate of
incorporation and the payment of required fees; no further
shareholder action is required. If issued, therights, preferences,
designations and limitations of such preferred stock would be set
by our Board and could operate to the disadvantage of the
outstanding common stock. Such terms could include, among others,
preferences as to dividends and distributions on liquidation. We
have designated a series of convertible preferred stock, the Series
A Convertible Preferred Stock. Each share of Series A Preferred
Stock is convertible, at the option of the holder thereof, at any
time after the issuance of such shareinto one (1) fully paid and
non-assessable share of Common Stock. Each outstanding share of
Series A Preferred Stock is entitled to one hundred (100) votes per
share on all matters to which the shareholders of the Corporation
are entitled or required to vote. As of the date hereof, there were
2,000,000 shares of Series A Convertible Preferred Stock issued and
outstanding.
Our officers and directors can sell some of their stock, which may
have a negative effect on our stock price and ability to raise
additional capital, and may make it difficult for investors to sell
their stock at any price.
Our
officers and directors, as a group, are the owners of 169,845
shares of our common stock, and with convertible preferred stock,
options and warrants to acquire another 3,570,600 shares of our
common stock, representing approximately 2% of our total issued and
outstanding shares of common stock. Each individual officer and
director may be able to sell up to 1% of our outstanding common
stock (currently approximately 1.8 million shares) every ninety
(90) days in the open market pursuant to Rule 144, which may have a
negative effect on our stock price and may prevent us from
obtaining additional capital. In addition, if our officers and
directors are selling their stock into the open market, it may make
it difficult or impossible for investors to sell their stock at any
price.
Our common stock is governed under The Securities Enforcement and
Penny Stock Reform Act of 1990.
The
Securities Enforcement and Penny Stock Reform Act of 1990 requires
additional disclosure relating to the market for penny stocks in
connection with trades in any stock defined as a penny stock. The
Commission has adopted regulations that generally define a penny
stock to be any equity security that has a market price of less
than $5.00 per share, subject to certain exceptions. Such
exceptions include any equity security listed on NASDAQ and any
equity security issued by an issuer that has (i) net tangible
assets of at least $2,000,000, if such issuer has been in
continuous operation for three years; (ii) net tangible assets
of at least $5,000,000, if such issuer has been in continuous
operation for less than three years; or (iii) average annual
revenue of at least $6,000,000, if such issuer has been in
continuous operation for less than three years. Unless an exception
is available, the regulations require the delivery, prior to any
transaction involving a penny stock, of a disclosure schedule
explaining the penny stock market and the risks associated
therewith.
SPECIAL NOTE ABOUT FORWARD-LOOKING STATEMENTS
We have
made forward-looking statements in this Annual Report, including
the sections entitled “Management’s Discussion and
Analysis of Financial Condition and Results of Operations”
and “Business,” that are based on our
management’s beliefs and assumptions and on information
currently available to our management. Forward-looking statements
include the information concerning our possible or assumed future
results of operations, business strategies, financing plans,
competitive position, industry environment, potential growth
opportunities, the effects of future regulation, and the effects of
competition. Forward-looking statements include all statements that
are not historical facts and can be identified by the use of
forward-looking terminology such as the words
“believe,” “expect,”
“anticipate,” “intend,” “plan,”
“estimate” or similar expressions. These statements are
only predictions and involve known and unknown risks and
uncertainties, including the risks outlined under “Risk
Factors” and elsewhere in this Annual Report.
Although we believe
that the expectations reflected in our forward-looking statements
are reasonable, we cannot guarantee future results, events, levels
of activity, performance or achievement. We are not under any duty
to update any of the forward-looking statements after the date of
this annual report to conform these statements to actual results,
unless required by law.
USE OF PROCEEDS
This
Prospectus relates to shares of our common stock that may be
offered and sold from time to time by the selling stockholders. We
will not receive any proceeds from the sale of shares of common
stock by the selling stockholders in this offering. We will pay for
expenses of this offering, except that the selling stockholders
will pay any broker discounts or commissions or equivalent expenses
applicable to the sale of their shares.
However, we will
receive up to $5,000,000 from the sale of common stock to Green
Coast Capital International SA under the Equity Purchase Agreement.
These proceeds would be received from time-to-time as Put Notices
are delivered to Green Coast, and we will use these proceeds for
working capital needs.
Our
allocation of proceeds represents our best estimate based upon the
expected requirements of our proposed business and marketing plan.
If any of these factors change, we may reallocate some of the net
proceeds. The portion of any net proceeds not immediately required
will be invested in certificates of deposit or similar short-term
interest bearing instruments.
INVESTMENT AGREEMENT
On
October 4, 2019, we entered into the Equity Purchase Agreement and
a Registration Rights Agreement with Green Coast Capital
International SA in order to establish a possible source of funding
for us.
Under the Equity Purchase Agreement, Green Coast
has agreed to provide us with up to $5,000,000 of funding upon effectiveness
of this prospectus; for which
1,000,000,000 shares of our common stock are being registered
pursuant to this prospectus. During this period, we can deliver a
put under the Equity Purchase Agreement by selling shares of our
common stock to Green Coast and Green Coast will be obligated to
purchase the shares. A put
transaction must close before
we can deliver another put notice to Green
Coast.
We may
request a put by sending a put notice to Green Coast, stating the
amount of the put. During the five trading days following a notice,
we will calculate the amount of shares we will sell to Green Coast
and the purchase price per share. The number of shares of Common
Stock that Green Coast shall purchase pursuant to each put notice
shall be determined by dividing the amount of the put by the
purchase price.
The
purchase price per share of common stock will be set at ninety
percent (90%) of the lowest closing
bid price of the common stock during the five consecutive trading
days immediately following the Clearing Date associated with
our Put Notice.
There
is no minimum amount we can put to Green Coast at any one time.
Upon effectiveness of the Registration Statement, the Company shall
deliver instructions to its transfer agent to issue shares of
Common Stock to Green Coast free of restrictive legends on or
before each closing date.
Pursuant to the Equity Purchase Agreement, Green
Coast and its affiliates shall not be issued shares of our common
stock that would result in its beneficial ownership
equaling more than 4.99% of our
outstanding common stock.
Green Coast will not enter into any short selling
or any other hedging activities during the pricing period.
On October 4, 2019, we entered into a
Registration Rights Agreement
with Green Coast requiring, among other things, that we prepare and
file with the SEC a Registration Statement on Form S-1 covering the
shares issuable to Green Coast
under the Equity Purchase Agreement. As per the Equity Purchase Agreement, none of
Green Coast’s obligations thereunder are transferrable and
may not be assigned to a third party.
SELLING SECURITY HOLDERS
The Selling Shareholder is Green Coast Capital
International SA, a Panama corporation and an underwriter in
this offering. Kevin Bobryk, President, has the sole voting and
dispositive power with respect to shares of stock beneficially
owned by Green Coast. Pursuant to the terms of an Equity Purchase
Agreement, at our election we may sell to Green Coast up to
$5,000,000 worth of our common stock at a price equal to ninety percent (90%) of the lowest
closing bid price of the common stock during the five consecutive
trading days immediately following the date of our notice to Green
Coast of our election to put shares pursuant to the Equity Purchase
Agreement.
In
connection with the Equity Purchase Agreement, we (i) issued to
Green Coast a convertible promissory note in the principal amount
of $150,000, for which they paid $25,000, and (ii) will pay to
Green Coast a cash fee of $10,000 out of the proceeds from the
first Put Notice.
As
of the date of this Prospectus, assuming a closing bid price of
$0.75 per share, our sales price to Green Coast would be $0.5625
per share and we would have to issue approximately 8,888,888 shares
of our common stock to receive all $5,000,000.
As
of the date of this Prospectus, there are approximately 186 million
shares of our common stock held by or currently issuable to
non-affiliates, representing approximately 99% of the outstanding
common stock prior to any sales to Green Coast. The 1,000,000,000
shares we are registering for resale by Green Coast represents
approximately 50% of the total authorized common
stock.
We
cannot sell shares to Green Coast if such shares would cause Green
Coast to own more than 4.99% of our common stock. As a result, as
of the date of this Prospectus, Green Coast cannot own more than
approximately 95 million shares after giving effect to that
issuance to Green Coast. If our total number of outstanding shares
of common stock increases, as it will as we sell shares to Green
Coast under the Equity Purchase Agreement, then we would be able to
sell more shares to Green Coast before reaching the 4.99%
threshold. In the event gross proceeds reach $5,000,000 from the
sale of less than 1,000,000,000 shares, the offering will end with
no further shares sold. Our limited trading volume and price
volatility is likely to inhibit Green Coast’s ability to
resell shares we sell to them, which will negatively impact our
ability to sell more shares to them. It is also likely that each
sale will decrease our stock price which means subsequent sale may
provide less proceeds per share that the previous sale. In
addition, we have only registered 1,000,000,000 shares for resale
by Green Coast.
Green
Coast intends to sell up to 1,000,000,000 shares and is an
“underwriter” within the meaning of the Securities Act
of 1933, as amended, in connection with the resale of our common
stock under the Equity Purchase Agreement. As of date of this
Prospectus, Green Coast or its affiliates owns zero shares of our
common stock prior to the offering. After the offering is
completed, unless they have sold some or all of the shares held as
of the date hereof, Green Coast will continue to own zero shares of
our common stock.
All of
the shares held by the selling stockholders are restricted
securities as that term is defined in Rule 144 promulgated under
the Securities Act of 1933.
PLAN OF DISTRIBUTION
The
Selling Shareholder of the common stock and any of their pledgees,
assignees and successors-in-interest may, from time to time, sell
any or all of their shares of common stock on the principal trading
market on which our common stock trades or any other stock
exchange, market or trading facility on which the shares are traded
or in private transactions. These sales may be at fixed or
negotiated prices. The Selling Shareholder may use any one or more
of the following methods when selling shares:
●
ordinary brokerage
transactions and transactions in which the broker-dealer solicits
purchasers;
●
block trades in
which the broker-dealer will attempt to sell the shares as agent
but may position and resell a portion of the block as principal to
facilitate the transaction;
●
purchases by a
broker-dealer as principal and resale by the broker-dealer for its
account;
●
privately
negotiated transactions;
●
broker-dealers may
agree with the Selling Shareholder to sell a specified number of
such shares at a stipulated price per share;
●
a combination of
any such methods of sale; or
●
any other method
permitted pursuant to applicable law.
The
Selling Shareholder may also sell shares under Rule 144 under the
Securities Act of 1933, as amended, if available, rather than under
this Prospectus.
Broker-dealers
engaged by the Selling Shareholder may arrange for other
brokers-dealers to participate in sales. Broker-dealers may receive
commissions or discounts from the Selling Shareholder (or, if any
broker-dealer acts as agent for the purchaser of shares, from the
purchaser) in amounts to be negotiated, but, except as set forth in
a supplement to this Prospectus, in the case of an agency
transaction not in excess of a customary brokerage commission in
compliance with FINRA Rule 2440; and in the case of a principal
transaction a markup or markdown in compliance with FINRA
IM-2440.
The Selling Shareholder is an underwriter within
the meaning of the Securities Act and any broker-dealers or agents
that are involved in selling the shares may be deemed to be
“underwriters” within the meaning of the Securities Act
in connection with such sales. In such event, any commissions
received by such broker-dealers or agents and any profit on the
resale of the shares purchased by them may be deemed to be
underwriting commissions or discounts under the Securities Act. The
Selling Shareholder has informed the Company that it does not have
any written or oral agreement or understanding, directly or
indirectly, with any person to distribute the Common Stock.
Pursuant to a requirement by the
Financial Industry Regulatory Authority, or FINRA, the maximum
commission or discount to be received by any FINRA member or
independent broker/dealer may not be greater than eight percent
(8%) of the gross proceeds received by us for the sale of any
securities being registered pursuant to SEC Rule 415 under the
Securities Act.
Discounts,
concessions, commissions and similar selling expenses, if any,
attributable to the sale of shares will be borne by the Selling
Shareholder. The Selling Shareholder may agree to indemnify
any agent, dealer or broker-dealer that participates in
transactions involving sales of the shares if liabilities are
imposed on that person under the Securities Act.
We will
pay all expenses in connection with the registration and sale of
the common stock by the Selling Shareholder. The estimated expenses
of issuance and distribution are set forth below:
|
Registration
Fees
|
Approximately
|
$13
|
|
Transfer Agent
Fees
|
Approximately
|
1,000
|
|
Costs of Printing
and Engraving
|
Approximately
|
1,000
|
|
Legal
Fees
|
Approximately
|
15,000
|
|
Accounting and
Audit Fees
|
Approximately
|
5,000
|
|
Total
|
|
$22,013
|
We
will not receive any proceeds from the resale of any of the shares
of our common stock by the Selling Shareholder. We may,
however, receive proceeds from the sale of our common stock under
the Equity Purchase Agreement. Neither the Equity Purchase
Agreement, nor any rights of the parties thereunder, may be
assigned or delegated to any other person.
Because
the Selling Shareholder is an “underwriter” within the
meaning of the Securities Act, it will be subject to the Prospectus
delivery requirements of the Securities Act including Rule 172
thereunder. In addition, any securities covered by this Prospectus
which qualify for sale pursuant to Rule 144 under the Securities
Act may be sold under Rule 144 rather than under this Prospectus.
There is no underwriter or coordinating broker acting in connection
with the proposed sale of the resale shares by the Selling
Shareholder.
We
agreed to keep this Prospectus effective until all the shares
covered by the registration statement of which this Prospectus is a
part (i) have been sold, thereunder or pursuant to Rule 144, or
(ii) (A) may be sold without volume or manner-of-sale restrictions
pursuant to Rule 144 and (B) (I) may be sold without the
requirement for us to be in compliance with the current public
information requirement under Rule 144 or (II) we are in compliance
with the current public information requirement under Rule 144, or
(iii) the commitment period under the Committed Equity Facility
Agreement has expired and no registrable securities are then held
of record by the Selling Shareholder that are subject to any resale
restriction under Rule 144, as determined by our counsel in a
written opinion letter to such effect, addressed and acceptable to
the transfer agent and the affected Selling Shareholder. The resale
shares will be sold only through registered or licensed brokers or
dealers if required under applicable state securities laws. In
addition, in certain states, the resale shares may not be sold
unless they have been registered or qualified for sale in the
applicable state or an exemption from the registration or
qualification requirement is available and is complied
with.
Under
applicable rules and regulations under the Exchange Act, any person
engaged in the distribution of the resale shares may not
simultaneously engage in market making activities with respect to
the common stock for the applicable restricted period, as defined
in Regulation M, prior to the commencement of the distribution. In
addition, the Selling Shareholder will be subject to applicable
provisions of the Exchange Act and the rules and regulations
thereunder, including Regulation M, which may limit the timing of
purchases and sales of shares of the common stock by the Selling
Shareholder or any other person. We will make copies of this
Prospectus available to the Selling Shareholder and have informed
them of the need to deliver a copy of this Prospectus to each
purchaser at or prior to the time of the sale.
The
Securities Enforcement and Penny Stock Reform Act of 1990 requires
additional disclosure relating to the market for penny stocks in
connection with trades in any stock defined as a penny stock. The
Commission has adopted regulations that generally define a penny
stock to be any equity security that has a market price of less
than $5.00 per share, subject to certain exceptions. Such
exceptions include any equity security listed on NASDAQ and any
equity security issued by an issuer that has (i) net tangible
assets of at least $2,000,000, if such issuer has been in
continuous operation for three years, (ii) net tangible assets
of at least $5,000,000, if such issuer has been in continuous
operation for less than three years, or (iii) average annual
revenue of at least $6,000,000, if such issuer has been in
continuous operation for less than three years. Unless an exception
is available, the regulations require the delivery, prior to any
transaction involving a penny stock, of a disclosure schedule
explaining the penny stock market and the risks associated
therewith.
DESCRIPTION OF SECURITIES
Our
authorized capital stock consists of 2,000,000,000 shares of common
stock, par value $0.00001, and 10,000,000 shares of preferred
stock, par value $0.001. As of November 12, 2019, there were
186,961,480 shares of our common stock issued and outstanding, and
2,000,000 shares of Series A Convertible Preferred Stock issued and
outstanding.
Common Stock. Each shareholder of our
common stock is entitled to a pro rata share of cash distributions
made to shareholders, including dividend payments. The holders of
our common stock are entitled to one vote for each share of record
on all matters to be voted on by shareholders. There is no
cumulative voting with respect to the election of our directors or
any other matter. Therefore, the holders of more than 50% of the
shares voted for the election of those directors can elect all of
the directors. The holders of our common stock are entitled to
receive dividends when and if declared by our Board of Directors
from funds legally available therefore. Cash dividends are at the
sole discretion of our Board of Directors. In the event of our
liquidation, dissolution or winding up, the holders of common stock
are entitled to share ratably in all assets remaining available for
distribution to them after payment of our liabilities and after
provision has been made for each class of stock, if any, having any
preference in relation to our common shareholders of shares of our
common stock have no conversion, preemptive or other subscription
rights, and there are no redemption provisions applicable to our
common stock.
On June
26, 2018, we conducted a reverse split of our common stock at a
ratio of 1-for-250 (the “Reverse Split”). All share numbers
in this prospectus reflect the effect of the reverse stock split of
our common stock on June 26, 2018.
Preferred Stock. We are authorized to
issue 10,000,000 shares of preferred stock, par value $0.001 per
share, of which 2,000,000 shares of Series A Convertible Preferred
stock have been authorized and issued. The Preferred Stock is
convertible, at the option of the holder, into one share of common
stock for each share of Preferred Stock converted. The holders of
our Preferred Stock also have 100 votes per share of Preferred
Stock that they hold, to be voted as a group along with the common
shareholders on all matters on which the shareholders are entitled
to vote. Other than these votes, the holders of our Preferred Stock
have no specific rights, as a group, to elect directors. The
holders of our preferred stock are not entitled to a dividend
preference over the common stock, but are entitled to a liquidation
preference in the amount of $1.25 per share. The preferred stock is
not redeemable. Finally, the holders of the preferred stock are
entitled to protective provisions as follows:
The
Company may not take any of the following actions without the
approval of a majority of the holders of the outstanding Series A
Convertible Preferred Stock: (i) effect a sale of all or
substantially all of the Company’s assets or which results in
the holders of the Company’s capital stock prior to the
transaction owning less than fifty percent (50%) of the voting
power of the Company’s capital stock after the transaction;
(ii) alter or change the rights, preferences, or privileges of
the Series A Convertible Preferred Stock; (iii) increase or
decrease the number of authorized shares of Series A Convertible
Preferred Stock; (iv) authorize the issuance of securities having a
preference over or on par with the Series A Convertible Preferred
Stock; or (v) effectuate a forward or reverse stock split or
dividend of the Company’s common stock.
An increase in our authorized
common stock has been approved by our shareholders and is
anticipated to be effective on or about December 19,
2019.
The
voting rights of the Series A Convertible Preferred Stock were not
affected by the Reverse Split, and as a result, the Series A
Convertible Preferred stockholders now have voting control of over
51% of our voting stock.
Dividend Policy. We have not declared or
paid a cash dividend on our capital stock in our last two fiscal
years and we do not expect to pay cash dividends on our common
stock in the foreseeable future. We currently intend to retain our
earnings, if any, for use in our business. Any dividends declared
in the future will be at the discretion of our Board of Directors
and subject to any restrictions that may be imposed by our
lenders.
Options and Warrants.
Mitchell S. Felder
owns outstanding warrants to acquire a total of 12,000 shares of
our common stock at $0.0025 per share.
There
are outstanding warrants to acquire 11,760 shares of our common
stock at $362.50 per share. Of these warrants, William A. Hartman
and Dr. Mitchell S. Felder each hold 620, Heidi Carl holds 480,
John S. Borza holds 4,480 and Jay Rosen holds 200. The remaining
4,880 warrants are held equally by Ramon D. Foltz, Scott Barnes and
Richard T. Najarian, former members of our Board of
Directors.
There
are outstanding warrants to acquire 44,800 shares of our common
stock at $62.50 per share. Of these warrants, William A. Hartman
and Dr. Mitchell S. Felder each hold 6,400, Heidi Carl holds 5,600,
John S. Borza holds 4,800, Dr. Patricio Reyes holds 2,800, Jay
Rosen holds 1,600, and three (3) investors own 17,200.
There
are outstanding warrants to acquire 2,000 shares of our common
stock at $50 per share held by one (1) investor.
There
are outstanding warrants to acquire 2,000 shares of our common
stock at $25 per share held by one (1) investor.
There
are outstanding warrants to acquire 22,200 shares of our common
stock at $12.50 per share. Of these warrants, William A. Hartman
and Dr. Mitchell S. Felder each holds 4,000, Heidi Carl holds
3,000, John S. Borza holds 2,400, Dr. Patricio Reyes holds 1,400,
Jay Rosen holds 800 and six (6) investors hold 6,600.
There
are outstanding 121,215 Series A Warrants to acquire shares of our
common stock at $7.5 per share and 121,215 Series B Warrants to
acquire shares of our common stock at $12.50 per share held by
three (3) investors.
There
are outstanding warrants to acquire 163,000 shares of our common
stock at $1.25 per share. Of these warrants, William A. Hartman and
Dr. Mitchell S. Felder each holds 34,000, Heidi Carl holds 24,000,
John S. Borza holds 29,000, Dr. Patricio Reyes holds 16,000, Jay
Rosen holds 4,000, John Pauly holds 8,000, and three (3) investors
hold 14,000.
Other
than as set forth above, as of the date of this prospectus, we do
not have any outstanding options, warrants, or other convertible
securities.
INTEREST OF NAMED EXPERTS AND COUNSEL
Clyde
Snow & Sessions, PC serves as our legal counsel in connection
with this offering. Clyde Snow & Sessions does not directly,
nor do any of its attorneys, own any shares of our common
stock.
DESCRIPTION OF BUSINESS
Corporate Information
We
were incorporated on May 10, 2010 in the State of Nevada. We have
two wholly-owned subsidiaries, Premier Biomedical Pain Relief Meds,
LLC, a Nevada limited liability company organized on September 14,
2017, and Health Stations, LLC, a Nevada limited liability company
organized on August 28, 2019.
Our
corporate headquarters are located in Jackson Center, PA. Our
mailing address is P.O. Box 25, Jackson Center, PA 16133,
and our telephone number is (724) 633-7033. We have offices
virtually in the homes of our management team who
reside in Pennsylvania, Michigan and various other states. Our
websites are www.premierbiomedical.com and
www.painreliefmeds.com.
Information contained on our website is not incorporated into, and
does not constitute any part of, this Annual Report.
Overview
We were
strictly a research-based company that intended to discover cures
for PTSD, cancer and various other diseases. In order to fund
on-going research and development in these areas, we developed a
line of topical hemp oil pain relief products. We began selling
these pain relief products in January of 2017 with a single product
and currently have nine topical pain relief products.
Through
our continued development and expansion of proprietary drugs and
treatments, we have reorganized the company into six technology
centers: (1) extra-corporeal treatment of disease, (2) PTSD
treatment, (3) anti-breast cancer drugs, (4) hemp oil/CBD pain
relief products, (5) anti-aging treatments, and (6) chemical and
alcohol addiction treatment.
In the
first quarter of 2017, initial sales of our pain management
products were made through a joint venture. In the third quarter of
2017, the joint venture was terminated and we began sales of our
pain management products directly from the Company.
Nature’s Pain Relief
We have not yet launched our latest brand,
Nature’s
Pain Relief, which will be
marketing 12 hemp oil products, including a 96-hour anti-pain
patch, three roll-on topical products, two sprays, two ointments,
two tincture drop products, a help oil capsule, and a
“doggie” pet product. All of these products will be
available through a new website at www.naturespainrelief.com.
Pain Management Products
We have
developed and are now marketing all-natural, hemp-oil based
products that are pesticide and solvent free. These products
provide generalized, neuropathic and localized topical pain
relief.
We
offer alternatives to dangerous and addictive opioid pain killers.
In the past year we have rapidly expanded our product offerings,
and we now offer nine pain relief products that are leaders in the
pain-relief field:
1.
96-hour pain relief
patch with 50 mg of hemp oil extract, the highest level of pain
relief ingredient available in the industry;
2.
120 mg/ 10 ml
water-based roll-on applicator;
3.
150 mg/ 10 ml
oil-based roll-on applicator;
4.
150 mg/ 30 ml
oil-based pump spray applicator;
5.
150 mg/ 2 oz.
ointment;
6.
200 mg/10 ml
oil-based roll-on applicator;
7.
500 mg/ 30 ml
oil-based pump spray applicator; and
8.
500 mg/ 1 oz.
ointment.
We
believe that this nine-product array positions us favorably in the
topical pain relief marketplace. The topical pain relief market is
expected to grow rapidly in the next few years, due to the focus on
reduction of opioid pain medication use, and we intend to be a
major player in that expanding market.
Now
that we have completed the product design and development phase, we
are aggressively embarking on the product distribution and sales
phase by:
1.
Expanding our
online sales beyond our web site at: www.painreliefmeds.com;
2.
Securing the
services of a social media coordinator to ensure that we optimize
that promotional tool;
3.
Recruiting a
National Sales Director to coordinate our growing field of sales
representatives and distributors;
4.
Securing the
services of a sales organization with expertise in marketing to the
government and senior care facilities;
5.
Engaging an
investor relations firm to facilitate television appearances
designed to gain optimum exposure for our company and its
products;
6.
Appearing in radio
and television broadcasts, and podcasts, via Uptick Newswire
periodically to ensure that our story gets out to the public;
and
7.
Retaining the
services of marketing firms to promote the Company and its products
through social media.
8.
Establishing
relationships with major distributors who will blanket specialized
sales outlets such as pharmacies, doctors’ offices,
convenience stores, long-term care facilities, large retail
facilities, etc.
In
addition, we are in the process of seeking potential partnerships
outside the United States to manufacture and market our products
worldwide. We anticipate that these partnerships will make new
markets available to us and allow us to rapidly increase our sales
and profitability through favorable manufacturing
arrangements.
Customers indicate
that they were able to achieve pain relief from our products and
stop the use of opioid painkillers. Public awareness of the harmful
side effects of opioid painkillers has grown significantly, and
many states have initiated litigation against drug makers claiming
they misrepresented the risks of opioid painkillers. As patients
seek to cut back their use of opioid painkillers and look for
alternatives, we believe demand for our products will see an
increase. We intend to petition national insurance agencies to urge
them to consider covering the use of our all-natural pain relief
products as a safe alternative to opioid painkillers.
Sales of our pain management products began on
February 1, 2017 through our former joint venture. Upon termination
of the joint venture, we began selling our products via our
website at www.painreliefmeds.com and
through various distributors. To
date, three pharmacies
and three chiropractic clinics have approved our products for sale and are
distributing our products. We anticipate that our products will
eventually be placed in several large pharmacy chains and sold in
several states.
Research and Development
We
intend to continue to discover and develop medical treatments for
humans, specifically targeting the pain management industry and the
treatment of:
|
-
|
Cancer
|
-
|
Fibromyalgia
|
|
-
|
Multiple
Sclerosis (MS)
|
-
|
Traumatic
Brain Injury (TBI)
|
|
-
|
Neuropathic
Pain
|
-
|
Alzheimer’s
Disease (AD)
|
|
-
|
Amyotrophic
Lateral Sclerosis
(ALS/Lou
Gehrig’s Disease)
|
-
|
Blood
Sepsis and Viremia
|
To
target cancer, Alzheimer’s disease, ALS, blood sepsis,
leukemia, and other life-threatening cancers, we intend to develop
our proprietary Sequential-Dialysis
Technique. The methodology involved in this technique is
largely unexplored and has been described by scientists as the
“wild west” of modern medicine. Consequently, our first
entry into the therapeutics market for medications that work
against cancer, multiple sclerosis, infectious diseases,
Alzheimer’s disease, strokes and traumatic brain injury
carries significant obstacles before reaching the opportunities of
a $700 billion industry.
Feldetrex®
We also
are in the process of developing our proprietary drug candidate
Feldetrex™, a
potential treatment for multiple sclerosis, fibromyalgia,
neuropathic pain and traumatic brain injury. The formulation used
in the current Feldetrex® will
be individually tailored to the various illnesses we intend to
target, with each formulation being given a unique proprietary
brand name. The annual market size of multiple sclerosis treatment
is $500 million and the annual market size for all proposed
Feldetrex®
market segments is $16 billion.
To
overcome the significant obstacles inherent to the development of
our Sequential-Dialysis
Technique and Feldetrex®
candidate drug, we are seeking to partner with prestigious
institutions and pharmaceutical companies with the substantial
infrastructure and resource capacity to perform experimentation and
to engage in product development in an inexpensive and efficient
manner.
Innovation
by our research and development operations is very important to our
success. Our goal is to discover, develop and bring to market
innovative products and treatments that address major unmet medical
needs, including initially, multiple sclerosis, septicemia, and
cancer. We expect this goal to be supported by substantial research
and development investments.
We
plan on conducting research internally and may also research
through contracts with third parties, through collaborations with
universities and biotechnology companies, and in cooperation with
pharmaceutical firms. We may also seek out promising compounds and
innovative technologies developed by third parties to incorporate
into our discovery or development methods and procedures or
projects, as well as our future product lines, through acquisition,
licensing or other arrangements.
In
addition to discovering and developing new products, methods and
procedures of treatment and treatments, we expect our research
operations to add value to our existing products and methods and
procedures of treatment in development by improving their
effectiveness and by discovering new uses for them.
Sequential-Dialysis Technique
Our
proprietary Sequential-Dialysis
Technique is a methodology for the removal of those
molecules which are harmful and responsible for causing diseases. A
significant disappointment in the practice of modern medicine is
that the capabilities do exist to eliminate the presence of most
illnesses, including life-threatening diseases such as AIDS and
cancer, but with a caveat that the process of treatment comes with
catastrophic side effects that can and often do kill the
patient.
Our
development is that the innovative Sequential-Dialysis
Methodology is done extracorporeally (outside the body).
This is a truly unique and innovative method for alleviating
disease.
We
believe that this methodology can be used for the prevention of
cancer metastasis, for directly attacking the causation of
intractable seizures, for preventing the death of anterior motor
neurons in ALS, for preventing the cause of the neuropathological
changes in Alzheimer’s disease and traumatic brain injury and
for eradicating the causations of infectious diseases, and our
intention is that the effectiveness of this technique will be
demonstrated and supported in future clinical studies.
Through
our Sequential-Dialysis
Technique, we ultimately hope to provide a cure for cancer
if not only to dramatically extend the lives of suffering patients.
Our initial focus is on lab and animal tests. Clinical trials, as
required, will be undertaken subsequently.
Feldetrex™
Although a
combination of generic medications, we have a U.S. Patent (No.
8,865,733) on our Feldetrex®
candidate drug. In this way, Feldetrex® is
similar to Viagra®, which was a proprietary cardiac drug prior
to its current use and ownership by Pfizer. Consequently, we have
one pending patent application for our Feldetrex®
candidate drug—intending to increase our Feldetrex®
related patent applications to three in the near
future.
Feldetrex® may
serve as an additional medication utilized by physicians for the
treatment of multiple sclerosis, fibromyalgia, or traumatic brain
injury, and is designed to decrease symptomatology in those
conditions. Feldetrex® will
not compete against our proprietary Sequential-Dialysis
Technique in the market to treat traumatic brain injury, but
rather the two will work conjunctively.
Feldetrex®
utilizes a low dosage of Naltrexone which has been shown in
multiple medical articles in the medical literature to increase
endogenous enkephalins4 (endogenous enkephalins are
pain-relieving pentapeptides produced in the body, located in the
pituitary gland, brain, and GI tract. Axon terminals that release
enkephalins are concentrated in the posterior horn of the gray
matter of the spinal cord, in the central part of the thalamus, and
inthe amygdala of the limbic system of the cerebrum. Endogenous
Enkephalins function as neurotransmitters that inhibit
neurotransmitters in the pathway for pain perception, thereby
reducing the emotional as well as the physical impact of pain). We
have not independently conducted medical or laboratory tests to
show the mechanism of action of this medication. While Naltrexone in high dosages acts
as an opioid antagonist, it inhibits opiate receptors.
Naltrexone in low dosages causes a compensatory upregulation
(increase in the number of receptors) of native endorphins and
enkephalins, which last beyond the effects of the Naltrexone
itself. We believe that this means, paradoxically, that a daily
dose of low dose Naltrexone can be used to chronically
increase endorphin and enkephalin levels. We believe that by
utilizing a low dosage, Naltrexone has a unique ability to
increase enkephalins and other neurotransmitters in the brainstem
of patients.
Marketing
Currently, we manage our marketing
responsibilities internally. Sales of our pain management products
are made primarily online through our website: www.painreliefmeds.com. We intend to seek
a partnership with and/or sale of our product
candidates/technologies to large
pharmaceutical and/or medical devices firms. These firms have the
ability to effectively promote our product candidates to healthcare
providers and patients. Through their marketing organizations, they
can explain the approved uses, benefits and risks of our product
candidates to healthcare providers such as doctors, nurse
practitioners, physician assistants, pharmacists, hospitals,
Pharmacy Benefit Managers (PBMs), Managed Care Organizations
(MCOs), employers and government agencies. They also market
directly to consumers in the U.S. through direct-to-consumer
advertising that communicates the approved uses, benefits, and
risks of our product candidates while continuing to motivate people
to have meaningful conversations with their doctors. In addition,
they sponsor general advertising to educate the public on disease
awareness, important public health issues, and patient assistance
programs.
A.
Bowling, Allen C..
"Low-dose naltrexone (LDN) The "411" on LDN" National Multiple
Sclerosis Society.
http://www.nationalmssociety.org/multimedia-library/momentum-magazine/back-issues/momentum-spring-09/index.aspx.
Retrieved 6 July 2011.
B.
Bourdette, Dennis.
"Spotlight on Low Dose Naltrexone (LDN)". US Department of Veteran
Affairs.
http://www.va.gov/MS/articles/Spotlight_on_Low_Dose_Naltrexone_LDN.asp.
Retrieved 5 July 2011.
C.
Giesser, Barbara S.
(2010). Primer on Multiple Sclerosis. New York: Oxford University
Press US. pp. 377. ISBN 978-0-19-536928-1.
D.
Moore, Elaine A.
1948. The promise of low dose naltrexone therapy: potential
benefits in cancer, autoimmune, neurological and infectious
disorders. Elaine A. Moore and Samantha Wilkinson. ISBN
978-0-7864-3715-3.
E.
Crain SM, Shen K-F
(1995). Ultra-low concentrations of naloxone selectively antagonize
excitatory effects of morphine on sensory neurons, thereby
increasing its antinociceptive potency and attenuating
tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci
USA 92: 10540–10544.
F.
Powell KJ,
Abul-Husn NS, Jhamandas A, Olmstead MC, Beninger RJ, et al. (2002).
Paradoxical effects of the opioid antagonist naltrexone on morphine
analgesia, tolerance, and reward in rats. J Pharmacol Exp Ther 300:
588–596.
G.
Wang H-Y, Friedman
E, Olmstead MC, Burns LH (2005). Ultra-low-dose naloxone suppresses
opioid tolerance, dependence and associated changes in Mu opioid
receptor-G protein
coupling and Gbc signaling; Neuroscience 135:
247–261.
The
large pharmaceutical/medical devices firms principally sell their
products to wholesalers, but they also sell directly to retailers,
hospitals, clinics, government agencies and pharmacies and also
work with MCOs, PBMs, employers and other appropriate healthcare
providers to assist them with disease management, patient education
and other tools that help their medical treatment
routines.
Patents and Intellectual Property Rights
We have
licensed three U.S. patents: Sequential Extracorporeal Treatment of
Bodily Fluids, U.S. Patent No. 9,216,386 and Utilization of Stents
for the Treatment of Blood Borne Carcinomas, U.S. Patent No.
8,758,287 (both from Marv Enterprises, LLC), and Medication and
Treatment for Disease, U.S. Patent No. 8,865,733 (from Altman
Enterprises, LLC), in the areas of cancer, sepsis, and multiple
sclerosis. We expect these patents to cover the medical treatments
discussed above for multiple sclerosis, blood sepsis, and cancer
and be effective until 2029. Marv and Altman have licensed these
technologies to us pursuant to the terms of license agreements.
Because our license agreements cover the patents and
“all applications of the United
States and foreign countries that claim priority to the above PCT
applications, including any non-provisionals, continuations,
continuations-in-part, divisions, reissues, re-examinations or
extensions thereof,” we anticipate that other
technologies that derive from these patents will also belong to us
and are covered by the license agreements.
Patents
extend for twenty years from the date of patent filing. The actual
protection afforded by a patent, which can vary from country to
country, depends upon the type of patent, the scope of its coverage
and the availability of legal remedies in the country.
Dr.
Felder is the owner of the Feldetrex mark, and has also licensed
this to us pursuant to the terms of a license
agreement.
We
expect our patent and related rights to be of material importance
to our business.
Competition
Our
business is conducted in an intensely competitive and often highly
regulated market. Our treatments face competition in the form of
branded drugs, generic drugs and the currently practiced treatments
for multiple sclerosis, blood sepsis, and cancer. The principal
forms of competition include efficacy, safety, ease of use, and
cost effectiveness. Where possible, companies compete on the basis
of the unique features of their products, such as greater efficacy,
better patient ease of use or fewer side effects. A lower overall
cost of therapy is also an important factor. Products that
demonstrate fewer therapeutic advantages must compete for inclusion
based primarily on price. Though the means of competition vary
among product categories, demonstrating the value of our
medications and procedures will be a critical factor for our
success.
Our
competitors include large worldwide research-based drug companies,
smaller research companies with more limited therapeutic focus, and
generic drug manufacturers. We compete with other companies that
manufacture and sell products that treat similar diseases as our
major medications and procedures.
Environment
Our
business may be subject to a variety of federal, state and local
environmental protection measures. We intend to comply in all
material respects with applicable environmental laws and
regulations.
Regulation
Pain Management Products
A major
obstacle to our growth is the public perception that hemp and
marijuana are the same thing. This perception drives much of the
regulation of hemp products. Although hemp and marijuana are both
part of the cannabis family, they differ in cultivation, function,
and application. Despite the use of marijuana becoming more widely
legalized, it is viewed by many regulators and many others as an
illegal product. Hemp, on the other hand, is used in a variety of
other ways that include clothing, skin products, pet products,
dietary supplements (the use of CBD oil), and thousands of other
applications. Hemp may be legally sold, however the inability of
many to understand the difference between hemp and marijuana often
causes burdensome regulation and confusion among potential
customers. Therefore, we are affected by laws related to cannabis
and marijuana, even though our products are not the direct targets
of these laws.
Cannabis is
currently a Schedule I controlled substance under the Controlled
Substance Act (“CSA”) and is, therefore, illegal under
federal law. Even in those states in which the use
of cannabis has been legalized pursuant to state law, its
use, possession and/or cultivation remains a violation of federal
law. A Schedule I controlled substance is defined as one that has
no currently accepted medical use in the United States, a lack of
safety for use under medical supervision and a high potential for
abuse. The U.S. Department of Justice (the “DOJ”)
describes Schedule I controlled substances as “the most
dangerous drugs of all the drug schedules with potentially severe
psychological or physical dependence.” If the federal
government decides to enforce the CSA in the states, persons that
are charged with distributing, possessing with intent to distribute
or growing cannabis could be subject to fines and/or
terms of imprisonment, the maximum being life imprisonment and a
$50 million fine.
Notwithstanding the
CSA, 29 U.S. states, the District of Columbia and the U.S.
territories of Guam and Puerto Rico allow their residents to use
medical cannabis. The states of Alaska, California, Colorado,
Maine, Massachusetts, Nevada, Oregon, Vermont (effective July 1,
2018) and Washington, and the District of Columbia, allow
cannabis for adult recreational use. Such state and
territorial laws are in conflict with the federal CSA, which
makes cannabis use and possession illegal at the federal
level.
In
light of such conflict between federal laws and state laws
regarding cannabis, the previous administration under
President Obama had effectively stated that it was not an efficient
use of resources to direct federal law enforcement agencies to
prosecute those lawfully abiding by state-designated laws allowing
the use and distribution of medical cannabis. For example, the
prior DOJ Deputy Attorney General of the Obama administration,
James M. Cole, issued a memorandum (the “Cole Memo”) to
all United States Attorneys providing updated guidance to federal
prosecutors concerning cannabis enforcement under the
CSA. In addition, the Financial Crimes Enforcement Network
(“FinCEN”) provided guidelines (the “FinCEN
Guidelines”) on February 14, 2014, regarding how financial
institutions can provide services to cannabis-related
businesses consistent with their Bank Secrecy Act
(“BSA”) obligations.
Additional existing
and pending legislation provides, or seeks to provide, protection
to persons acting in violation of federal law but in compliance
with state laws regarding cannabis. The Rohrabacher-Blumenauer
Amendment (formerly known as the Rohrbacher-Farr Amendment) to the
Commerce, Justice, Science and Related Agencies Appropriations
Bill, which funds the DOJ, since 2014 has prohibited the DOJ from
using funds to prevent states with laws authorizing the use,
distribution, possession or cultivation of
medical cannabis from implementing such laws. On August
2016, the Ninth Circuit Court of Appeals ruled in United States v. McIntosh that the
Amendment bars the DOJ from spending funds on the prosecution of
conduct that is allowed by state medical cannabis laws,
provided that such conduct is in strict compliance with applicable
state law. The Rohrabacher-Blumenauer Amendment is currently
effective through September 30, 2018, but as an amendment to an
appropriations bill, it must be renewed annually.
These
developments previously were met with a certain amount of optimism
in the cannabis industry, but (i) neither the CARERS Act
nor the Respect State Marijuana Laws Act of 2017 have yet been
adopted, (ii) the Rohrabacher-Blumenauer Amendment, being an
amendment to an appropriations bill that must be renewed annually,
has not currently been renewed beyond September 30, 2018, and (iii)
the ruling in United States
v. McIntosh is only applicable precedent in the Ninth
Circuit.
Because
of the discrepancy between the laws in some states, which permit
the distribution and sale of medical and
recreational cannabis, from federal law that prohibits any
such activities, DOJ Deputy Attorney General James M. Cole issued
the Cole Memo concerning cannabis enforcement under the
CSA.
At the
time of its issuance, the Cole Memo reiterated Congress’s
determination that cannabis is a dangerous drug and that
the illegal distribution and sale of cannabis is a
serious crime that provides a significant source of revenue to
large-scale criminal enterprises, gangs, and cartels. The Cole Memo
noted that the DOJ was committed to enforcement of the CSA
consistent with those determinations. It also noted that the DOJ
was committed to using its investigative and prosecutorial
resources to address the most significant threats in the most
effective, consistent, and rational way. In furtherance of those
objectives, the Cole Memo provided guidance to DOJ attorneys and
law enforcement to focus their enforcement resources on persons or
organizations whose conduct interferes with any one or more of the
following important priorities (the “Enforcement
Priorities”) in preventing:
●
the distribution
of cannabis to minors;
●
revenue from the
sale of cannabis from going to criminal enterprises,
gangs, and cartels;
●
the diversion
of cannabis from states where it is legal under state law
in some form to other states;
●
state-authorized cannabis activity
from being used as a cover or pretext for the trafficking of other
illegal drugs or other illegal activity;
●
violence and the
use of firearms in the cultivation and distribution
of cannabis;
●
drugged driving and
the exacerbation of other adverse public health consequences
associated with cannabis use;
●
the growing
of cannabis on public lands and the attendant public
safety and environmental dangers posed
by cannabis production on public lands; and
●
cannabis possession
or use on federal property.
However, on January
4, 2018, the U.S. Attorney General, Jeff Sessions, issued a
memorandum for all U.S. Attorneys (the “Sessions Memo”)
stating that the Cole Memo was rescinded effective immediately. In
particular, Mr. Sessions stated that “prosecutors should
follow the well-established principles that govern all federal
prosecutions,” which require “federal prosecutors
deciding which cases to prosecute to weigh all relevant
considerations, including federal law enforcement priorities set by
the Attorney General, the seriousness of the crime, the deterrent
effect of criminal prosecution, and the cumulative impact of
particular crimes on the community.” The Sessions Memo went
on to state that given the DOJ’s well-established general
principles, “previous nationwide guidance specific to
marijuana is unnecessary and is rescinded, effective
immediately.”
It is
unclear at this time whether the Sessions Memo indicates that the
Trump administration will strongly enforce the federal laws
applicable to cannabis or what types of activities will
be targeted for enforcement. However, a significant change in the
federal government’s enforcement policy with respect to
current federal laws applicable to cannabis could cause
significant financial damage to us. We do not currently cultivate,
distribute or sell cannabis, but our hemp oil products are
closely tied to the cannabis industry.
Although the
Sessions Memo has rescinded the Cole Memo and it is unclear at this
time what the ultimate impact of that rescission will have on our
business, if any, we intend to continue to conduct rigorous due
diligence to verify the legality of all activities that we engage
in and ensure that our activities do not interfere with any of the
Enforcement Priorities set forth in the Cole Memo.
On
March 26, 2018, Senate Majority Leader Mitch McConnell, R-Kentucky,
announced plans to introduce The Hemp Farming Act of 2018 to
exclude hemp from the CSA. The proposed bill would legalize hemp as
an agricultural commodity and remove it from the list of controlled
substances. This would greatly reduce the uncertainty we face with
respect to regulation of hemp products. However, Congress has not
yet taken formal action to pass the bill.
Other Medical Products
The
development of proprietary drugs and medications is subject to
varying degrees of governmental regulation in the United States and
any other countries in which our operations are conducted. In the
United States, regulation by various federal and state agencies has
long been focused primarily on product safety, efficacy,
manufacturing, advertising, labeling and safety reporting. The
exercise of broad regulatory powers by the U.S. Federal Drug
Administration (“FDA”) continues to result in increases
in the amounts of testing and documentation required for FDA
clearance of new drugs and devices and a corresponding increase in
the expense of product introduction. Likewise, the approval process
with the FDA is estimated to take approximately seven (7) years
from the time it is started. Similar trends are also evident in
major markets outside of the United States.
Clinical
trials are a set of procedures in medical
research conducted to allow safety (or more specifically,
information about adverse drug reactions and adverse
effects of other treatments) and efficacy data to be
collected for health interventions (e.g., drugs, diagnostics,
devices, therapy protocols). These trials can take place only after
satisfactory information has been gathered on the quality of the
non-clinical safety, and Health Authority/Ethics
Committee approval is granted in the country where the trial
is taking place.
Depending on the
type of product and the stage of its development, investigators
enroll healthy volunteers and/or patients into small pilot
studies initially, followed by larger scale studies in
patients that often compare the new product with the currently
prescribed treatment. As positive safety and efficacy data are
gathered, the number of patients is typically increased. Clinical
trials can vary in size from a single center in one country to
multicenter trials in multiple countries.
Due to
the sizable cost a full series of clinical trials may incur, the
burden of paying for all the necessary people and services is
usually borne by the sponsor who may be a governmental
organization, a pharmaceutical,
or biotechnology company. Since the diversity of roles
may exceed resources of the sponsor, often a clinical trial is
managed by an outsourced partner such as a contract
research organization or a clinical trials unit in the
academic sector.
The
regulatory agencies under whose purview we intend to operate have
administrative powers that may subject us to such actions as
product withdrawals, recalls, seizure
of products and other civil and criminal
sanctions.
Because we intend to seek a partnership with
and/or sale of our product candidates/technologies to large
pharmaceutical and/or medical devices firms, we anticipate that a
larger pharmaceutical company will undertake to navigate the
regulatory pathway, including conducting clinical trials, for a
product such as Feldetrex™.
Employees
As of
the date hereof, we do not have any employees other than our
officers and directors. Our officers and directors will continue to
work for us for the foreseeable future. We anticipate hiring
appropriate personnel on an as-needed basis, and utilizing the
services of independent contractors as needed.
DESCRIPTION OF PROPERTY
We do
not currently lease or use any office space. We have not paid any
amounts to Mr. Hartman for the use of his personal office or for
reimbursement of personal office expenses incurred by
him.
LEGAL PROCEEDINGS
We are
not a party to or otherwise involved in any legal
proceedings.
In the
ordinary course of business, we are from time to time involved in
various pending or threatened legal actions. The litigation process
is inherently uncertain and it is possible that the resolution of
such matters might have a material adverse effect upon our
financial condition and/or results of operations. However, in the
opinion of our management, other than as set forth herein, matters
currently pending or threatened against us are not expected to have
a material adverse effect on our financial position or results of
operations.
SELECTED FINANCIAL DATA
|
|
As
of and for the Nine Months ended September 30,
|
As
of and for the Year Ended December 31,
|
As
of and for the Year Ended December 31,
|
|
Premier
Biomedical, Inc.
|
|
|
|
|
|
|
|
|
|
Statement of
Operations Data:
|
|
|
|
|
|
|
|
|
|
Revenue
|
$12,975
|
$39,795
|
$39,761
|
|
Net operating
income (loss)
|
$(219,688)
|
$(692,842)
|
$(1,289,838)
|
|
Net income
(loss)
|
$(340,171)
|
$(398,886)
|
$(3,763,558)
|
|
|
|
|
|
|
|
|
|
|
|
Balance Sheet
Data:
|
|
|
|
|
|
|
|
|
|
Cash
|
$117,209
|
$86,827
|
$83,704
|
|
Current
assets
|
$177,582
|
$159,787
|
$203,603
|
|
Total
assets
|
$185,500
|
$164,990
|
$209,081
|
|
|
|
|
|
|
Current
liabilities
|
$2,303,710
|
$2,312,382
|
$2,819,807
|
|
Total
liabilities
|
$2,303,710
|
$2,312,382
|
$2,819,807
|
|
Accumulated
deficit
|
$(17,067,869)
|
$(16,727,698)
|
$(16,328,812)
|
|
|
|
|
|
|
Net loss per common
share – basic and diluted
|
$(0.02)
|
$(0.11)
|
$(0.92)
|
Our cash and total current assets remained relatively steady as we
continued to sustain losses funded by the sale of convertible
notes. Our total current liabilities decreased primarily due to the
reduction of our accounts payable of $66,718 and the conversion of
outstanding convertible notes of $177,423, offset by a net increase
in accrued interest of $27,049 and the change in the value of our
derivative liabilities of $148,348, along with $338,300 of
additional debt financing, net of debt discounts of $278,228. Our
stockholders’ deficit decreased by $29,182 to
($2,118,210).
MANAGEMENT’S DISCUSSION AND ANALYSIS OR PLAN OF
OPERATION
Disclaimer Regarding Forward Looking Statements
You
should read the following discussion in conjunction with our
financial statements and the related notes and other financial
information included in this Form S-1. In addition to historical
financial information, the following discussion contains
forward-looking statements that reflect our plans, estimates and
beliefs. Our actual results could differ materially. Factors that
could cause or contribute to these differences include those
discussed below and elsewhere in this Form S-1, particularly in the
Section titled Risk Factors.
Although the
forward-looking statements in this registration statement reflect
the good faith judgment of our management, such statements can only
be based on facts and factors currently known by them.
Consequently, and because forward-looking statements are inherently
subject to risks and uncertainties, the actual results and outcomes
may differ materially from the results and outcomes discussed in
the forward-looking statements. You are urged to carefully review
and consider the various disclosures made by us in this report and
in our other reports as we attempt to advise interested parties of
the risks and factors that may affect our business, financial
condition, and results of operations and prospects.
The
following discussion and analysis of financial condition and
results of operations of the Company is based upon, and should be
read in conjunction with, its audited and unaudited financial
statements and related notes elsewhere in this Form S-1, which have
been prepared in accordance with accounting principles generally
accepted in the United States.
Summary Overview
We were
strictly a research-based company that intended to discover cures
for PTSD, cancer and various other diseases. In order to fund
on-going research and development in these areas, we developed a
line of topical hemp oil pain relief products. We began selling
these pain relief products in January of 2017 with a single product
and currently have nine topical pain relief products.
Through
our continued development and expansion of proprietary drugs and
treatments, we have reorganized the company into six technology
centers: (1) extra-corporeal treatment of disease, (2) PTSD
treatment, (3) anti-breast cancer drugs, (4) hemp oil/CBD pain
relief products, (5) anti-aging treatments, and (6) chemical and
alcohol addiction treatment.
Pain Management Products
We have
developed and are now marketing all-natural, hemp-oil based
products that are pesticide and solvent free. These products
provide generalized, neuropathic and localized topical pain
relief.
We
offer alternatives to dangerous and addictive opioid pain killers.
In the past year we have rapidly expanded our product offerings,
and we now offer nine pain relief products that are leaders in the
pain-relief field:
1.
96-hour pain relief
patch with 50 mg of hemp oil extract, the highest level of pain
relief ingredient available in the industry;
2.
120 mg/ 10 ml
water-based roll-on applicator;
3.
150 mg/ 10 ml
oil-based roll-on applicator;
4.
150 mg/ 30 ml
oil-based pump spray applicator;
5.
150 mg/ 2 oz.
ointment;
6.
200 mg/10 ml
oil-based roll-on applicator;
7.
500 mg/ 30 ml
oil-based pump spray applicator; and
8.
500 mg/ 1 oz.
ointment.
We
believe that this eight-product array positions us favorably in the
topical pain relief marketplace. The topical pain relief market is
expected to grow rapidly in the next few years, due to the focus on
reduction of opioid pain medication use, and we intend to be a
major player in that expanding market.
Now
that we have completed the product design and development phase, we
are aggressively embarking on the product distribution and sales
phase by:
1.
Expanding our
online sales beyond our web site at: www.painreliefmeds.com;
2.
Securing the
services of a social media coordinator to ensure that we optimize
that promotional tool;
3.
Recruiting a
National Sales Director to coordinate our growing field of sales
representatives and distributors;
4.
Securing the
services of a sales organization with expertise in marketing to the
government and senior care facilities;
5.
Engaging an
investor relations firm to facilitate television appearances
designed to gain optimum exposure for our company and its
products;
6.
Appearing in radio
and television broadcasts, and podcasts, via Uptick Newswire
periodically to ensure that our story gets out to the public;
and
7.
Retaining the
services of marketing firms to promote the Company and its products
through social media.
8.
Establishing
relationships with major distributors who will blanket specialized
sales outlets such as pharmacies, doctors’ offices,
convenience stores, long-term care facilities, large retail
facilities, etc.
In
addition, we are in the process of seeking potential partnerships
outside the United States to manufacture and market our products
worldwide. We anticipate that these partnerships will make new
markets available to us and allow us to rapidly increase our sales
and profitability through favorable manufacturing
arrangements.
Customers indicate
that they were able to achieve pain relief from our products and
stop the use of opioid painkillers. Public awareness of the harmful
side effects of opioid painkillers has grown significantly, and
many states have initiated litigation against drug makers claiming
they misrepresented the risks of opioid painkillers. As patients
seek to cut back their use of opioid painkillers and look for
alternatives, we believe demand for our products will see an
increase. We intend to petition national insurance agencies to urge
them to consider covering the use of our all-natural pain relief
products as a safe alternative to opioid painkillers.
Financing
In the
past, as we worked through the development of our products, we have
relied heavily on financing through various issuances of common
stock, warrants and convertible debt. As our sales grow, we expect
to find financing solutions in the future that help us expand our
operations, avoid dilution to our shareholders, and ultimately
increase our company valuation.
Through
the remainder of 2019, we will continue to market our pain
management products and seek a wider distribution network through
the negotiation of distribution agreements with large pharmacy
chains, military branches, government agencies, senior care
facilities and international partners.
Through
our reorganization into six technology centers, we are positioned
to take advantage of opportunities to individually sell, license or
commercialize the technologies produced within each of these
centers to suitable investment partners, without dilutive equity
issuances. In the long run, we believe that this will be most
beneficial to our investors.
Going Concern
As a
result of our current financial condition, we have received a
report from our independent registered public accounting firm for
our financial statements for the years ended December 31, 2018 and
2017 that includes an explanatory paragraph describing the
uncertainty as to our ability to continue as a going concern. In
order to continue as a going concern, we must effectively balance
many factors and generate more revenue so that we can fund our
operations from our sales and revenues. If we are not able to do
this, we may not be able to continue as an operating company.
During the nine months ended September 30, 2019, we completed the sale of convertible notes to raise $
308,400 of net proceeds from several investors. We cannot be
sure that sources of capital will be available to us for the
remainder of 2019 and into 2020. However, without additional
capital in the short term, we may not be able to push forward in
the production and marketing of our new pain management products.
Until we are able to grow revenues sufficient to meet our operating
expenses, we must continue to raise capital by issuing debt or
through the sale of our stock. There is no assurance that our cash
flow will be adequate to satisfy our operating expenses and capital
requirements.
Results of Operations for the Three and Nine Months Ended September
30, 2019 and 2018
Introduction
We had
revenues of $3,465 and $12,975 for the three and nine months ended
September 30, 2019, respectively, compared to $8,225 and $30,709
for the three and nine months ended September 30, 2018,
respectively. This was a decrease of $9,510, or 73%, for the three
months ended September 30, 2019 compared to 2018, and $22,484, or
73%, for the nine months ended September 30, 2019 compared to
2018.
Our
operating expenses were $66,366 and $227,193 for the three and nine
months ended September 30, 2019, respectively, compared to $85,185
and $240,834 for the three and nine months ended September 30,
2018, respectively. This was a decrease of $18,819, or 22%, for the
three months ended September 30, 2019 compared 2018, and a decrease
of $13,641, or 6%, for the nine months ended September 30, 2019
compared to 2018.
Our
results of operations for the three and nine months ended September
30, 2019 and 2018 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
$3,465
|
$8,225
|
$12,975
|
$30,709
|
|
Cost of goods
sold
|
1,653
|
4,373
|
5,470
|
20,577
|
|
Gross
profit
|
1,812
|
3,852
|
7,505
|
10,132
|
|
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
|
|
General and
administrative
|
36,757
|
65,572
|
138,589
|
139,881
|
|
Professional
fees
|
29,609
|
22,613
|
88,604
|
100,953
|
|
Total operating
expenses
|
66,366
|
85,185
|
227,193
|
240,834
|
|
|
|
|
|
|
|
Net operating
loss
|
(64,554)
|
(81,333)
|
(219,688)
|
(230,702)
|
|
Other income
(expense)
|
(117,080)
|
(282,202)
|
(120,483)
|
333,485
|
|
|
|
|
|
|
|
Net income
(loss)
|
$(181,634)
|
$(363,535)
|
$(340,171)
|
$102,783
|
Revenues
The
Company was established on May 10, 2010, and began to generate
revenues during the third quarter of 2017 from the sale of pain
patches made with CBD oils. Our sales are comprised of both website
sales to individual consumers and brick and mortar pharmacies, and
we have expanded from patches to oils, sprays, and roll-ons. Our
cost of goods sold primarily consists of the products and the
packaging. The decrease in our revenues for the three and nine
months ended September 30, 2019, compared to three and nine months
ended September 30, 2018, while significant in percentage terms,
was not material in absolute terms and reflects the timing of
product sales.
Cost of Goods Sold
Cost of
goods sold for the three and nine
months ended September 30, 2019 were $1,653, or 48% of
sales, and $5,470, or 42% of sales, compared to $4,373, or 53% of
sales, and $20,577, or 67% of sales, for the three and nine months
ended September 30, 2018. Cost of sales consists primarily of
product materials and packaging supplies. Our cost of goods sold as
a percentage of sales was reduced because of lower prices in the
marketplace and slightly higher volume pricing.
General and Administrative
General
and administrative expenses were $36,757 and $138,589,
respectively, for the three and nine months ended September 30,
2019, compared to $62,572 and $139,881, respectively, for the three
and nine months ended September 30, 2018, a decrease of $25,815 and
$1,292, or 41% and less than 1%, respectively. The decrease was
primarily due to decreased investor relations and advertising
expenses.
Professional Fees
Professional fees expense was $29,609 and $88,604,
respectively, for the three and nine months ended September 30,
2019, compared to $22,613 and $100,953, respectively, for the three
and nine months ended September 30, 2018, an increase of $6,996 and
a decrease of $12,349, or 31% and 12%, respectively.
Professional fees consist primarily of legal and, accounting and
auditing services.
Net Operating Loss
Net
operating loss was $64,554 and $219,688, respectively, for the
three and nine months ended September 30, 2019, compared to $81,333
and $230,702, respectively, for the three and nine months ended
September 30, 2018, a decrease of $16,779 and $11,014, or 21% and
5%, respectively. The net operating loss decreased during the nine
months ended September 30, 2019 because of decreased operating
expenses as previously described.
Other Income/Expense
Other
income (expense) was ($117,080) and ($120,483), respectively, for
the three and nine months ended September 30, 2019, compared to
($282,202) and $333,485, respectively, for the three and nine
months ended September 30, 2018, a decrease in expenses of $165,122
and $453,968, respectively. Other expense for the nine months ended
September 30, 2019 consisted of interest expense of $101,793 and a
change in derivative liabilities of $18,690. Other expense for the
nine months ended September 30, 2018 consisted of interest
expense of $322,323 offset by a gain of $655,808 in market value of
derivative liabilities.
Net Income (Loss)
Net
income (loss) for the three and nine months ended September 30,
2019, was ($181,634) or ($0.01) per share, and ($340,171) or
($0.02) per share, respectively, compared to ($363,535) or ($0.11)
per share, and $102,783 or $0.03 per share, respectively, for the
three and nine months ended September 30, 2018. Net loss changes,
as set forth above, were primarily due to the change in derivative
liabilities.
Liquidity and Capital Resources as of and for the nine months ended
September 30, 2019 and the year ended December 31,
2018.
Introduction
During
the three and nine months ended September 30, 2019, we had negative
operating cash flows. Our cash on hand as of September 30, 2019 was
$117,209, which was derived from the sale of convertible promissory
notes to investors. Our monthly cash flow burn rate for the first
nine months of 2018 was approximately $35,000, and our monthly burn
rate through the nine months ended September 30, 2019 was
approximately $30,000. Although we have moderate short term cash
needs, as our operating expenses increase as we ramp up production
and sales of our new products we will face strong medium to long
term cash needs. We anticipate that these needs will be satisfied
through the issuance of debt or the sale of our securities until
such time as our cash flows from operations will satisfy our cash
flow needs.
Our
cash, current assets, total assets, current liabilities, and total
liabilities as of September 30, 2019 and December 31, 2018,
respectively, are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
$117,209
|
$86,827
|
$30,382
|
|
Total Current
Assets
|
177,582
|
159,787
|
17,795
|
|
Total
Assets
|
185,500
|
164,990
|
20,510
|
|
Total Current
Liabilities
|
2,303,710
|
2,312,382
|
(8,672)
|
|
Total
Liabilities
|
$2,303,710
|
$2,312,382
|
$(8,672)
|
Our
cash and total current assets remained relatively steady as we
continued to sustain losses funded by the sale of convertible
notes. Our total current liabilities decreased primarily due to the
reduction of our accounts payable of $66,718 and the conversion of
outstanding convertible notes of $177,423, offset by a net increase
in accrued interest of $27,049 and the change in the value of our
derivative liabilities of $148,348, along with $338,300 of
additional debt financing, net of debt discounts of $278,228. Our
stockholders’ deficit decreased by $29,182 to
($2,118,210).
In
order to repay our obligations in full or in part when due, we will
be required to raise significant capital from other sources. There
is no assurance, however, that we will be successful in these
efforts.
Cash Requirements
Our
cash on hand as of September 30, 2019 was $117,209. Based on our
minimal revenues and current monthly burn rate of approximately
$30,000 per month, we will need to continue to fund operations by
raising capital from the sale of our stock and debt
financings.
Sources and Uses of Cash
Operations
We had
net cash used in operating activities of ($273,168) for the nine
months ended September 30, 2019, compared to ($318,471) for the
nine months ended September 30, 2018. This decrease in net cash
used in operating activities was a result of an increase in the
fair market value of derivative liabilities of $674,498 and an
increase in inventory of $39,861, offset by a decrease in
amortization of debt discounts of $246,886.
Investments
We had
$4,850 net cash used in investing activities for the nine months
ended September 30, 2019, and $2,029 net cash used in investing
activities for the nine months ended September 30, 2018. We
outsource the manufacturing of our products, so our investment
expenses are minimal.
Financing
Our net
cash provided by financing activities for the nine months ended
September 30, 2019 was $308,400, which consisted of the proceeds
from the sale of convertible notes. For the nine months ended
September 30, 2018, net cash provided by financing activities was
$300,000, which consisted of the proceeds from the sale of
convertible notes.
Results of Operations for the Years Ended December 31, 2018 and
2017
Introduction
We had
revenues of $39,795 and $39,761 for the years ended December 31,
2018 and 2017, respectively. Our operating expenses were $618,910
for the year ended December 31, 2018 compared to $1,304,160 for the
year ended December 31, 2017, an increase of $685,250, or 53%. Our
operating expenses consisted of research and development costs,
general and administrative expenses, and professional fees,
including $296,944 and $783,404 of stock-based compensation for the years ended
December 31, 2018 and 2017, respectively.
Revenues and Net Operating Loss
Our
revenues, operating expenses, and net operating loss for the years
ended December 31, 2018 and 2017 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
$39,795
|
$39,761
|
$34
|
|
Cost of goods
sold
|
113,727
|
25,439
|
88,288
|
|
Gross profit
(loss)
|
(73,932)
|
14,322
|
(88,254)
|
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
|
Research and
development
|
-
|
184,315
|
(184,315)
|
|
General and
administrative
|
189,285
|
196,670
|
(7,385)
|
|
Professional
fees
|
429,625
|
923,175
|
(493,550)
|
|
Total operating
expenses
|
618,910
|
1,304,160
|
(685,250)
|
|
|
|
|
|
|
Net operating
loss
|
(692,842)
|
(1,289,838)
|
(596,996)
|
|
Other income
(expense)
|
293,956
|
(2,473,720)
|
2,767,676
|
|
|
|
|
|
|
Net
loss
|
$(398,886)
|
$(3,763,558)
|
$(3,364,672)
|
Revenues
The
Company was established on May 10, 2010, and began initial sales of
our pain management products during the year ended December 31,
2017.
Research and Development
Research
and development expenses were $-0- for the year ended December 31,
2018 compared to $184,315 for the year ended December 31, 2017, an
increase of $184,315. The expenses decreased due to final invoices
from the University of Texas at El Paso. We plan to continue to
reduce our research and development activities as we focus on our
pain management products.
General and Administrative
General
and administrative expenses were $189,285 for the year ended
December 31, 2018 as compared to $196,670 for the year ended
December 31, 2017, a decrease of $7,385, or 4%.
Professional Fees
Professional
fees expense was $429,625 for the year ended December 31, 2018,
compared to $923,175 for the year ended December 31, 2017, a
decrease of $493,550, or 53%. The decrease was primarily due to the
decrease of stock-based compensation issued to debt holders,
directors and consultants for services rendered. A total of
$296,944 of stock-based compensation was awarded during the year
ended December 31, 2018, compared to $783,404 of stock-based
compensation during the year ended December 31, 2017, a decrease of
$486,460 from 2017 to 2018. The decrease in stock-based
compensation was the result of $671,424 of shares of common stock
that were awarded to note holders during 2017.
Net Operating Loss
Net
operating loss for the year ended December 31, 2018 was $692,842,
compared to a net operating loss of $1,289,838 for the year ended
December 31, 2017, a decrease of $596,996, or 46%. Net operating
loss decreased, as set forth above, primarily due to a decrease in
stock-based compensation issued to note holders for services
rendered.
Other Income (Expense)
Other
income (expense) for the year ended December 31, 2018 was $293,956,
compared to ($2,473,720) for the year ended December 31, 2017, a
net increase of $2,767,676, or 112%. Other income (expense)
consisted of interest and finance charges on debt and equity
financing, gain on early extinguishment of debt, and a change in
the fair value of derivative liabilities during the year ended
December 31, 2018. Other income (expense) consisted of interest and
finance charges on debt and equity financing, a change in the fair
value of derivative liabilities, as well as a net loss on our joint
venture during the year ended December 31, 2017. The net increase
was primarily due to an increase of approximately $2,818,479 in the
value of derivative liabilities related to significant decreased
convertible debt financing during the year ended December 31, 2018,
compared to the year ended December 31, 2017.
Net Loss
Net
loss for the year ended December 31, 2018 was $398,886, or $(0.11)
per share, compared to a net loss of $3,763,558, or $(1.92) per
share, for the year ended December 31, 2017, a decrease of
$3,364,672, or 89%. Net loss decreased, as set forth above,
primarily due to an increase of approximately $2,818,479 in the
value of derivative liabilities and decreased stock-based
compensation issued to note holders for services
rendered.
Liquidity and Capital Resources as of and for the Nine Months ended
September 30, 2019 and 2017
Introduction
During
the three and nine months ended September 30, 2019, we had negative
operating cash flows. Our cash on hand as of September 30, 2019 was
$117,209, which was derived from the sale of convertible promissory
notes to investors. Our monthly cash flow burn rate for the first
nine months of 2018 was approximately $35,000, and our monthly burn
rate through the nine months ended September 30, 2019 was
approximately $30,000. Although we have moderate short term cash
needs, as our operating expenses increase as we ramp up production
and sales of our new products we will face strong medium to long
term cash needs. We anticipate that these needs will be satisfied
through the issuance of debt or the sale of our securities until
such time as our cash flows from operations will satisfy our cash
flow needs.
Our
cash, current assets, total assets, current liabilities, and total
liabilities as of September 30, 2019 and December 31, 2018,
respectively, are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
$117,209
|
$86,827
|
$30,382
|
|
Total Current
Assets
|
177,582
|
159,787
|
17,795
|
|
Total
Assets
|
185,500
|
164,990
|
20,510
|
|
Total Current
Liabilities
|
2,303,710
|
2,312,382
|
(8,672)
|
|
Total
Liabilities
|
$2,303,710
|
$2,312,382
|
$(8,672)
|
Our
cash and total current assets remained relatively steady as we
continued to sustain losses funded by the sale of convertible
notes. Our total current liabilities decreased primarily due to the
reduction of our accounts payable of $71,855 and the conversion of
outstanding convertible notes of $95,075, offset by an increase in
accrued interest of $27,049 and the value of our derivative
liabilities of $148,348. Our stockholders’ deficit decreased
by $29,182 to ($2,118,210).
In
order to repay our obligations in full or in part when due, we will
be required to raise significant capital from other sources. There
is no assurance, however, that we will be successful in these
efforts.
Cash Requirements
Our
cash on hand as of September 30, 2019 was $117,209. Based on our
minimal revenues and current monthly burn rate of approximately
$30,000 per month, we will need to continue to fund operations by
raising capital from the sale of our stock and debt
financings.
Sources and Uses of Cash
Operations
We had
net cash used in operating activities of ($273,168) for the nine
months ended September 30, 2019, compared to ($318,471) for the
nine months ended September 30, 2018. This decrease in net cash
used in operating activities was a result of an increase in the
fair market value of derivative liabilities of $674,498 and an
increase in inventory of $39,861, offset by a decrease in
amortization of debt discounts of $246,886.
Investments
We had
$4,850 net cash used in investing activities for the nine months
ended September 30, 2019, and $2,029 net cash used in investing
activities for the nine months ended September 30, 2018. We
outsource the manufacturing of our products, so our investment
expenses are minimal.
Financing
Our net
cash provided by financing activities for the nine months ended
September 30, 2019 was $308,400, which consisted of the proceeds
from the sale of convertible notes. For the nine months ended
September 30, 2018, net cash provided by financing activities was
$300,000, which consisted of the proceeds from the sale of
convertible notes.
Liquidity and Capital Resources as of and for the Years ended
December 31, 2018 and 2018
Introduction
During
the year ended December 31, 2018, because we did not generate any
revenues, we had negative operating cash flows. Our cash on hand as
of December 31, 2018 was $86,827, which was derived from the sale
of convertible promissory notes to investors. Our monthly cash flow
burn rate has increased from approximately $37,000 in 2018 to
approximately $42,500 in 2017. Although we have moderate short-term
cash needs, as our operating expenses increase we will face strong
medium to long-term cash needs. We anticipate that these needs will
be satisfied through the issuance of debt or the sale of our
securities until such time as our cash flows from operations will
satisfy our cash flow needs.
Our
cash, current assets, total assets, current liabilities, and total
liabilities as of December 31, 2018 and December 31, 2017,
respectively, are as follows:
|
|
|
|
|
|
|
|
|
|
|
Cash
|
$86,827
|
$83,704
|
$3,123
|
|
Total Current
Assets
|
159,787
|
203,603
|
(43,816)
|
|
Total
Assets
|
164,990
|
209,081
|
(44,091)
|
|
Total Current
Liabilities
|
2,312,382
|
2,819,807
|
(507,425)
|
|
Total
Liabilities
|
$2,312,382
|
$2,819,807
|
$(507,425)
|
Our
cash increased by $3,123 as of December 31, 2018, compared to
December 31, 2017. Our total current assets decreased by $43,816
primarily because we recognized an $87,650 allowance for inventory
obsolescence in 2018. Our total assets decreased by $44,091
primarily for the same reasons.
Our
current liabilities decreased by $507,425 as of December 31, 2018,
compared to December 31, 2017, primarily due to decreases in
accounts payable of $97,854 and derivative liabilities of $565,477.
Our total liabilities decreased by the same amount for the same
reasons as we do not have long term liabilities.
In
order to repay our obligations in full or in part when due, we will
be required to raise significant capital from other sources. There
is no assurance, however, that we will be successful in these
efforts.
Cash Requirements
Our
cash on hand as of December 31, 2018 was $86,827, which was derived
from the sale of convertible promissory notes and common stock. Our
monthly cash flow burn rate is approximately $37,000. Although we
have moderate short-term cash needs, as our operating expenses
increase we will face strong medium to long term cash needs. We
anticipate that these needs will be satisfied through the sale of
our securities until such time as our cash flows from operations
will satisfy our cash flow needs.
Sources and Uses of Cash
Operations
Our net
cash used in operating activities for the years ended December 31,
2018 and 2017 was $444,878 and $507,116, respectively, a decrease
of $62,238, or 12%. The primary uses of our cash were purchasing
inventory and operating our pain management business, along with
the public company compliance costs.
Investments
Our net
cash used in investing activities for the years ended December 31,
2018 and 2017 was $2,029 and $2,694, respectively, a decrease of
$665. The slight decrease reflected a lack of purchases of property
and equipment in 2018 compared to 2017.
Financing
Our net
cash provided by financing activities for the years ended December
31, 2018 and 2017 was $450,030 and $573,393, respectively, a
decrease of $123,363, or 22%. The decrease was primarily a result
of a decrease in proceeds from the sale of stock of $135,000 in
2018, compared to $285,000 of proceeds from the sale of stock in
2017 and a decrease in repayments of convertible notes payable from
$30,000 to zero. This was offset by a decrease in the proceeds from
the sale of stock on equity line of credit from $18,323 to
$-0-.
Securities Purchase Agreement
On
March 30, 2017, we entered into a Securities Purchase Agreement
with three investors and raised $300,000 through the sale of stock
and warrants. These same investors purchased $150,000 of common
stock and warrants in the second tranche on May 30, 2017.On August
8, 2017, we exchanged convertible notes with the investors for the
warrants issued in the first tranche and the common stock issued in
the second tranche. We also amended the Securities Purchase
Agreement on that date, and on October 30, 2017, the investors
purchased an additional $150,000 of our convertible notes. We
expect these investments, our growing revenues and sales of common
stock, warrants and convertible notes will finance our operations
for the next several months as we seek to expand revenues from our
new pain management products.
Sale of Convertible Notes
On March 1, 2018, we received $60,000 from two
investors from the sale of convertible notes. These investors have
also committed to provide an additional $240,000 in the near
future. The investors purchased convertible notes at the
signing of a Securities Purchase Agreement for an aggregate amount
of $60,000. The investors will buy additional convertible notes for
an aggregate of $60,000 within five trading days of our filing a
registration statement to cover the investors’ shares of
common stock issuable upon conversion of the convertible notes.
Within five trading days of the registration statement being
declared effective, the investors will buy additional convertible
notes for an aggregate of $180,000. For further details, see our
Form 8-K filed on March 5, 2018 and copies of the agreements filed
herewith as Exhibits 10.60, 10.61 and 10.62.
Critical Accounting Policies and Estimates
See
Note 1 to the Financial Statements for the year ended December 31,
2018 on page F-6 which is incorporated herein by
reference.
CHANGES IN AND DISAGREEMENTS WITH
ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
We have
no disclosure required by this item.
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS, AND CONTROL
PERSONS
The
following table sets forth the names, ages, and biographical
information of each of our current directors and executive
officers, and the positions with the Company held by each person,
and the date such person became a director or executive officer of
the Company. Our executive officers are elected annually by the
Board of Directors. The directors serve one-year terms until their
successors are elected. The executive officers serve terms of one
year or until their death, resignation or removal by the Board of
Directors. Family relationships among any of the directors and
officers are described below.
|
Name
|
|
Age
|
|
Position(s)
|
|
|
|
|
|
|
|
William
A. Hartman
|
|
77
|
|
President,
Chief Executive Officer, and Director (June 2010)
|
|
|
|
|
|
|
|
Dr.
Mitchell S. Felder
|
|
65
|
|
Chairman
of the Board of Directors and the Scientific Advisory Board (June
2010)
|
|
|
|
|
|
|
|
Heidi
H. Carl
|
|
49
|
|
Chief Financial Officer, Secretary, Treasurer and Director (June
2010)
|
|
|
|
|
|
|
|
Dr.
Patricio F. Reyes
|
|
72
|
|
Chief Technology Officer and Director (August 2016)
|
|
|
|
|
|
|
|
John S.
Borza
|
|
65
|
|
Vice President and Director (August 2012)
|
|
|
|
|
|
|
|
Jay
Rosen
|
|
65
|
|
Director (June 2010)
|
|
|
|
|
|
|
|
John
Pauly
|
|
58
|
|
Director (December 2017)
|
William A. Hartman, age 77, is our
President and Chief Executive Officer and a member of our Board of
Directors. From March 2008 until June 2010, Mr. Hartman was not
directly employed but was planning the formation of Premier
Biomedical, Inc. From October 2006 to March 2008, Mr. Hartman was
the Chief Operating Officer of Nanologix, Inc. From July 1991 to
July 2000, Mr. Hartman was a Director at TRW Automotive. From 1984
to 1991, Mr. Hartman was Chief Engineer at TRW Automotive and from
1979 to 1984 he was Division Quality Compliance Manager at Ford
Motor Company. At TRW Automotive, Mr. Hartman was one of the auto
industry pioneers of the concept of grouping related components
into systems and modules and shipping just-in-time to the vehicle
assembly plants. He founded and headed a separate business group
within TRW Automotive with plants in the U.S., Mexico and Europe
with combined annual sales of $1.3 Billion. Academic credentials
include a BSME degree from Youngstown State University and a MSIA
degree (Industrial Administration/Management) from the University
of Michigan.
Dr. Mitchell S.
Felder, age 65, is our Chairman
of the Board of Directors and our Scientific Advisory Board.
Dr. Felder is a practicing Board Certified Neurologist. Dr. Felder
acquired a B.A. Degree from the University of Pennsylvania in 1975
and an M.D. Degree from the University of Rome, Faculty of Medicine
in 1983. He has been Board Certified by both the American Academy
of Clinical Neurology and the American Board of Psychiatry and
Neurology. Dr. Felder has authored or co-authored six publications,
three studies, and has 18 issued patents. Dr. Felder is the former
President, Chairman, and founder of Infectech/Nanologix (from its
founding in 1989 through March 2007)—growing the company from
startup to a $100 million market cap. During the past five years,
Dr. Felder has had as his principal occupation and employment work
as an attending neurologist. Dr. Felder is presently an attending
neurologist at the William Beaumont Army Medical Center in El Paso,
Texas. Dr. Felder has more than 20 years of management
experience.
Heidi H.
Carl, age 49, is our Chief
Financial Officer, Secretary, Treasurer, and a member of our
Board of Directors. From May
2009 until June 2010, Ms. Carl was not directly employed but was
working with Mr. Hartman in planning the formation of Premier
Biomedical, Inc. From June 2007 to May
2009, Ms. Carl was the Product Development Specialist at General
Motors Corporation. From May 2006 to May 2007, Ms. Carl was the
Associate Marketing Manager at General Motors Corporation. From May
2003 to May 2006, Ms. Carl was the Marketing Specialist at General
Motors Corporation and from May 1999 to May 2003, Ms. Carl was the
District Area Parts Manager at General Motors Corporation. Academic
credentials include a BSBA degree from Madonna University and an
ASBA degree from Oakland Community College.
Dr. Patricio F. Reyes, MD, FAAN,
age 72, is a board certified neurologist and neuropathologist, has
served as the Chief Medical Officer and Board Member of the Retired
National Football League Players Association since 2013.
Dr. Reyes has been a board member of the Association of
Ringside Physicians since 2008 and was previously its Chair of the
Education Committee and 2009 Distinguished Educator.
Dr. Reyes has worked as a neurologist and
neuropathologist for the Phoenix VA Hospital since 2014.
Dr. Reyes is the co-founder, Chief Medical Officer and
Chair of the Scientific Advisory Board of Yuma Therapeutics, Inc.
where he has worked since 2012. He is a Fellow of the American
Academy of Neurology and was former Professor of Neurology and
Neuropathology at Thomas Jefferson Medical School in Philadelphia,
Pennsylvania, and Professor of Neurology, Pathology and Psychiatry
at Creighton University School of Medicine in Omaha,
Nebraska.
John S.
Borza, PE, AVS, age 65, is our
Vice President and was appointed to our Board of Directors on
August 17, 2012. Mr. Borza is currently the President and Chief
Executive Officer of Value Innovation, LLC, a consulting firm
focused on value engineering and creative problem solving, where he
has served since August 2009. Prior to Value Innovation, Mr. Borza
was a Specialist with TRW Automotive from September 2007 to
September 2009, and a Director at TRW Automotive from May 1999 to
September 2007. Earlier in his career, Mr. Borza worked in
R&D for 12 years on a variety of products and technologies in
various capacities ranging from Engineer to Chief Engineer, before
moving into launch and production support roles. Mr. Borza is an
Altshuller Institute certified TRIZ Practitioner, and a SAVE
International certified Associate Value Specialist. He is active in
the local chapter of SAVE International and currently serves as the
chapter Past-President. Mr. Borza holds a BS degree in Electrical
Engineering and an MBA from the University of
Michigan.
Jay Rosen, age 65, has been a member of
our Board of Directors since our inception in June 2010. Mr. Rosen
has been a partner at Rosen
Associates, a real estate holding and management company, since
1971. He is also a partner at Midway Industrial Terminal, a real
estate holding and management company, and has been since 2005. Mr.
Rosen privately owns and manages the Rosen Farm, cellular towers
and various other real estate properties, is the President of
XintCorp, a small start-up company for developing intellectual
property, and is a former member of the NY Mercantile Exchange and
the New York Futures Exchange. Mr. Rosen studied economics and
finance at New York University and Columbia
University.
John Pauly, age 58, has served as Executive Vice
President at HealthWarehouse.com, Inc., an online Verified Internet
Pharmacy Practice Site (VIPPS), since October 2017. From January
2017 through March 2017, Mr. Pauly served as the interim Chief
Executive Officer of HealthWarehouse.com. From January 2016 until
his time at HealthWarehouse.com, he was the Chief Operating Officer
of Specialty Medical Drug Store, also a VIPPS accredited online
pharmacy business.
Since
January 2014, Mr. Pauly has been an independent consultant and
investor to businesses in the pharmaceutical industry. From August
2013 through December 2013, he was Vice President at Acton
Pharmaceuticals where he was responsible for the strategy and
operations to commercialize its first FDA approved product,
handling all implementation activities through outsourced
third-parties.
From
January 2013 through August 2013, Mr. Pauly was a consultant to the
Chief Executive Officer of Crown Laboratories, Inc., a fully
integrated specialty pharmaceutical company. There he assisted in
creating and implementing corporate strategy, and OTC and Rx drug
development, manufacturing and commercialization.
Prior
to his time at Crown Laboratories, Mr. Pauly was at Merz
Pharmaceuticals, LLC, a specialty healthcare company and subsidiary
of Merz Pharmaceuticals GmbH, where he served as Vice President of
Commercial Operations from May 2011 to June 2012 and Executive
Director of OCG Strategy and Operations from March 2009 to April
2011. His duties at Merz included management of a unit of 98 people
covering a wide-range of business functions. He played a major role
in corporate strategy and evaluating licensing and M&A
opportunities.
Mr. Pauly has also served in a variety of management positions
within the healthcare and pharmaceutical industries since 1990 and
brings a wide range of skills and expertise to the Board of
Directors.
Family Relationships
Heidi
H. Carl is the daughter of William A. Hartman.
Other Directorships; Director Independence
Other
than as set forth above, none of our officers and directors is a
director of any company with a class of securities registered
pursuant to section 12 of the Exchange Act or subject to the
requirements of section 15(d) of such Act or any company registered
as an investment company under the Investment Company Act of
1940.
For
purposes of determining director independence, we have applied the
definitions set out in NASDAQ Rule 5605(a)(2). Neither the OTC Pink
Tier on which shares of common stock are quoted, nor the Pink
Sheets Current tier, has any director independence requirements.
The NASDAQ definition of “Independent Officer” means a
person other than an Executive Officer or employee of the Company
or any other individual having a relationship which, in the opinion
of the Company's Board of Directors, would interfere with the
exercise of independent judgment in carrying out the
responsibilities of a director. According to the NASDAQ definition,
only Mr. Rosen and Mr. Pauly are an independent
directors.
Board Committees
Our
Board of Directors does not maintain a separate audit, nominating
or compensation committee. Functions customarily performed by such
committees are performed by its Board of Directors as a whole. We
are not required to maintain such committees under the applicable
rules of the OTC Markets. We do not currently have an “audit
committee financial expert” since we currently do not have an
audit committee in place. We intend to create board committees,
including an independent audit committee, in the near future. We
have a Scientific Advisory Board that serves an advisory role
to management and the Board of Directors.
We do not currently
have a process for security holders to send communications to the
Board.
During
the fiscal years ended December 31, 2018 and 2017, the Board of
Directors met approximately on a bi-weekly basis. Only Mr. Rosen
attended fewer than 75 percent of the meetings. We have no policy
with regard to attendance at meetings of the Board of
Directors.
Involvement in Certain Legal Proceedings
None of
our officers or directors has, in the past ten years, filed
bankruptcy, been convicted in a criminal proceeding or named in a
pending criminal proceeding, been the subject of any order,
judgment, or decree of any court permanently or temporarily
enjoining him or her from any securities activities, or any other
disclosable event required by Item 401(f) of Regulation
S-K.
Code of Ethics
We have
not adopted a written code of ethics, primarily because we believe
and understand that our officers and directors adhere to and follow
ethical standards without the necessity of a written
policy.
Scientific Advisory Board
Our
Board of Directors has established a Scientific Advisory Board
that assists management and the Board of Directors on an advisory
basis with respect to the research, development, clinical,
regulatory and commercial plans and activities relating to
research, manufacture, use and sale of our pain management products
and drug candidates. The Scientific Advisory Board meets
on an ad hoc basis and
may attend meetings of the Board at the Board’s
request. Its current members are Mitchell S. Felder, MD,
Patricio F. Reyes, MD, FAAN, Carl E. Meyer, DO, MBA, and Kathryn
Meyer, DO. All members of the Scientific Advisory Board are doctors
and have extensive knowledge, experience and training in the fields
of medicine relevant to our business.
Scientific Advisory
Board members are not entitled to receive compensation, but
warrants or other benefits may be awarded at the discretion of the
Board of Directors. We granted warrants, to purchase 2,000 shares of our common
stock at an exercise price of $1.25 over seven (7) years from the
grant date on December 22, 2017, to each of Carl Meyer and
Katherine Meyer for their services on the Scientific Advisory
Board.
EXECUTIVE COMPENSATION
Narrative Disclosure of Executive Compensation
Effective on
September 28, 2012, we entered into employment agreements with our
President and Chief Executive Officer, William A. Hartman, and our
Chairman of the Board of Directors and Chairman of the Scientific
Advisory Board, Dr. Mitchell S. Felder. In December 2012, the
Company and Dr. Felder agreed to terminate his employment
agreement, effective as of its date of inception.
Pursuant to the
employment agreement with Hartman, he will be compensated in the
amount of $150,000 per year for the duration of the agreement.
Pursuant to the agreement, Hartman has waived the salary and the
accrual thereof in exchange for being issued a Common Stock
Purchase Warrant whereby Hartman may purchase a maximum of 105,000
shares of our common stock at a purchase price of $1.45 per share.
The agreement has a one-year term and provides for two (2) years of
severance at his then-current salary in the event Hartman is
terminated due to death, disability or without cause. Mr. Hartman
is still employed under the terms of the agreement.
We do
not currently have a written employment agreement with our other
executives. All other executives are at-will employees or
consultants whose compensation is set forth in the Summary
Compensation Table below.
Summary Compensation Table
The
following table sets forth information with respect to compensation
earned by our Chief Executive Officer, President, and Chief
Financial Officer for the years ended December 31, 2018 and
2017.
Name and
Principal Position
|
|
|
|
|
|
Non-Equity
Incentive Plan Compensation ($)
|
Nonqualified
Deferred Compensation ($)
|
All
Other
Compensation
($)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2018
|
-0-
|
-0-
|
-0-
|
68,146(2)
|
-0-
|
-0-
|
-0-
|
68,146
|
Chief
Executive Officer (1)
|
2017
|
-0-
|
-0-
|
-0-
|
23,358(3)
|
-0-
|
-0-
|
-0-
|
23,358
|
|
|
|
|
|
|
|
|
|
|
|
|
2018
|
|
|
|
|
|
|
|
|
|
Chief Financial
Officer(4)
|
2017
|
-0-
|
-0-
|
-0-
|
16,488(4)
|
-0-
|
-0-
|
-0-
|
16,488
|
(1)
Mr. Hartman does
not receive a salary for his services as Chief Executive
Officer.
(2)
Option awards
consist of warrants to purchase
842,000 shares of our common stock at an exercise price of $0.05
over seven (7) years from the grant date on December 15,
2018.
(3)
Option awards
consist of warrants to purchase 34,000
shares of our common stock at an exercise price of $1.25 over seven
(7) years from the grant date on December 22,
2017.
(4)
Ms. Carl does not
receive a salary for her services as Secretary and
Treasurer.
(5)
Option awards
consist of warrants to purchase
500,000 shares of our common stock at an exercise price of $0.09
over seven (7) years from the grant date on December 15,
2018.
(6)
Option awards
consist of warrants to purchase 24,000
shares of our common stock at an exercise price of $1.25 over seven
(7) years from the grant date on December 22,
2017.
Officer and Director Compensation
On
December 20, 2018, we issued warrants to the following officers and
directors, which will allow them to purchase shares of our common
stock in the amounts indicated: William Hartman (842,000 shares);
Mitchell Felder (842,000 shares), Heidi Carl (500,000 shares), John
Borza (579,000 shares), Patricio Reyes (500,000 shares), John Pauly
(52,500 shares) and Jay Rosen (52,500 shares). We also issued
warrants to purchase shares of our common stock to three members of
our Scientific Advisory Board in the amounts indicated: Heleno
Souza (131,000 shares), Carl Meyer (26,000 shares) and Katherine
Meyer (26,000 shares). The exercise price of the foregoing warrants
is Nine Cents ($0.09) per share. The warrants also have a cashless
exercise option.
The
warrants were issued with respect to services provided in 2018 and
vested immediately upon issuance. The issuance of the warrants was
fully approved by our Board of Directors on December 20, 2018, the
date a fully executed resolution authorizing the issuance was
delivered to us.
On
December 22, 2017, we issued warrants to the following officers and
directors, which will allow them to purchase shares of our common
stock in the amounts indicated: William Hartman (8,500,000 shares);
Mitchell Felder (8,500,000 shares), Heidi Carl (6,000,000 shares),
John Borza (7,250,000 shares), Patricio Reyes (4,000,000 shares)
and Jay Rosen (1,000,000 shares). We also issued warrants to
purchase shares of our common stock to three members of our
Scientific Advisory Board in the amounts indicated: Heleno Souza
(2,500,000 shares), Carl Meyer (500,000 shares) and Katherine Meyer
(500,000 shares). The exercise price of the foregoing warrants is
One Half Cent ($0.005) per share. The warrants also have a cashless
exercise option.
The
warrants were issued with respect to services provided in 2017 and
vested immediately upon issuance. The issuance of the warrants was
fully approved by our Board of Directors on December 22, 2017, the
date a fully executed resolution authorizing the issuance was
delivered to us.
Also on
December 22, 2017, we issued a warrant to John Pauly as an initial
incentive award following his appointment to the Board of
Directors, which will allow him to purchase 2,000,000 shares of our
common stock. The terms of this warrant are the same as those in
the warrants issued to the other directors on this date, having an
exercise price of One Half Cent ($0.005) and a cashless exercise
option.
We
do not currently have an established policy to provide compensation
to members of our Board of Directors for their services in that
capacity. We intend to develop such a policy in the near
future.
Outstanding Equity Awards at Fiscal Year-End
We
do not currently have a stock option or grant plan.
SECURITY OWNERSHIP OF CERTAIN
BENEFICIAL OWNERS AND MANAGEMENT
The
following table sets forth, as of November 7, 2019, certain
information with respect to the Company’s equity securities
owned of record or beneficially by (i) each Officer and Director of
the Company; (ii) each person who owns beneficially more than 5% of
each class of the Company’s outstanding equity securities;
and (iii) all Directors and Executive Officers as a
group.
|
|
|
Percentage of
Common Stock Ownership (2)
|
Series A
Preferred Stock Ownership
|
Percentage of
Series A Preferred Stock Ownership (3)
|
|
|
|
|
|
|
|
William A. Hartman
(4)(10)
|
971,020(8)
|
1.50%
|
1,000,000
|
50.0%
|
|
|
|
|
|
|
|
Dr. Mitchell S.
Felder (4)
(5)
|
968,051(9)
|
1.50%
|
1,000,000
|
50.0%
|
|
|
|
|
|
|
|
Heidi H. Carl
(4)(10)
|
537,080(12)
|
*
|
-
|
-
|
|
|
|
|
|
|
|
Jay Rosen
(4)
|
63,120(13)
|
*
|
-
|
-
|
|
|
|
|
|
|
|
John S. Borza
(4)
|
619,934(11)
|
*
|
-
|
-
|
|
|
|
|
|
|
|
John
Pauly(4)
(6)
|
60,500(15)
|
*
|
-
|
-
|
|
|
|
|
|
|
|
Dr. Patricio
Reyes(4)
(7)
|
520,680(14)
|
*
|
-
|
-
|
|
|
|
|
|
|
|
All Officers and
Directors as a Group (7 Persons)
|
3,740,445(8)(9)(11)(12)(13)(14)(15)
|
5.56%
|
2,000,000
|
100.0%
|
(1)
Unless otherwise
indicated, the address of the shareholder is c/o Premier
Biomedical, Inc.
(2)
Unless otherwise
indicated, based on 63,723,186 shares of common stock issued and
outstanding. Shares of common stock subject to options or warrants
currently exercisable, or exercisable within 60 days, are deemed
outstanding for purposes of computing the percentage of the person
or group holding such options or warrants, but are not deemed
outstanding for purposes of computing the percentage of any other
person.
(3)
Unless otherwise
indicated, based on 2,000,000 shares of Series A Convertible
Preferred Stock issued and outstanding.
(4)
Indicates one of
our officers or directors.
(5)
Dr. Felder’s
address is P.O. Box 1332, Hermitage, PA 16148.
(6)
Mr. Pauly’s
address is 900 Squire Oaks Drive, Villa Hills, KY
41017.
(7)
Dr. Reyes’
address is 10618 North Eleventh Place, Phoenix, AZ
85020.
(8)
Includes 4,000
shares of common stock that may be acquired upon the conversion of
1,000,000 shares of Series A Convertible Preferred Stock, 200
shares of common stock that may be acquired at $362.50 per share,
420 shares of common stock that may be acquired at $362.50 per
share if the Company’s common stock reaches a closing bid
price of $750.00 per share and remains at or above $750.00 per
share for thirty (30) consecutive trading days on any and all
markets or exchanges on which the Company’s common stock is
traded, 6,400 shares of common stock that may be acquired upon the
exercise of warrants at $62.50 per share, 4,000 shares of common
stock that may be acquired upon the exercise of warrants at $12.50
per share, 34,000 shares of common stock that may be acquired upon
the exercise of warrants at $1.25 per share, and 842,000 shares of
common stock that may be acquired upon the exercise of warrants at
$0.09 per share.
(9)
Includes 12,000
shares of common stock that may be acquired upon the exercise of
warrants at $0.0025 per share, 4,000 shares of common stock that
may be acquired upon the conversion of 1,000,000 shares of Series A
Convertible Preferred Stock, 200 shares of common stock that may be
acquired at $362.50 per share, 420 shares of common stock that may
be acquired at $362.50 per share if the Company’s common
stock reaches a closing bid price of $750.00 per share and remains
at or above $750.00 per share for thirty (30) consecutive trading
days on any and all markets or exchanges on which the
Company’s common stock is traded, 6,400 shares of common
stock that may be acquired upon the exercise of warrants at $62.50
per share, 4,000 shares of common stock that may be acquired upon
the exercise of warrants at $12.50 per share, 34,000 shares of
common stock that may be acquired upon the exercise of warrants at
$1.25 per share, and 579,000 shares of common stock that may be
acquired upon the exercise of warrants at $0.09 per
share.
(10)
William A. Hartman
is the father of Heidi H. Carl. Mr. Hartman disclaims ownership of
shares held by his daughter.
(11)
Includes 110 shares
of common stock owned members of Mr. Borza’s household, 4,200
shares of common stock that may be acquired by Mr. Borza at $362.50
per share upon the exercise of warrants, 280 shares of common stock
that may be acquired at $362.50 per share upon the exercise of
warrants if the Company’s common stock reaches a closing bid
price of $750.00 per share and remains at or above $750.00 per
share for thirty (30) consecutive trading days on any and all
markets or exchanges on which the Company’s common stock is
traded, 4,800 shares of common stock that may be acquired upon the
exercise of warrants at $62.50 per share, 2,400 shares of common
stock that may be acquired upon the exercise of warrants at $12.50
per share, 29,000 shares of common stock that may be acquired upon
the exercise of warrants at $1.25 per share, and 579,000 shares of
common stock that may be acquired upon the exercise of warrants at
$0.09 per share.
(12)
Includes 200 shares
of common stock that may be acquired upon the exercise of warrants
at $362.50 per share, 280 shares of common stock that can be
acquired at $362.50 per share if the Company’s common stock
reaches a closing bid price of $750.00 per share and remains at or
above $750.00 per share for thirty (30) consecutive trading days on
any and all markets or exchanges on which the Company’s
common stock is traded, 5,600 shares of common stock that may be
acquired upon the exercise of warrants at $62.50 per share, 3,000
shares of common stock that may be acquired upon the exercise of
warrants at $12.50 per share, 24,000 shares of common stock that
may be acquired upon the exercise of warrants at $1.25 per share,
and 500,000 shares of common stock that may be acquired upon the
exercise of warrants at $0.09 per share.
(13)
Includes 200 shares
of common stock that may be acquired upon the exercise of warrants
at $362.50 per share, 1,600 shares of common stock that may be
acquired upon the exercise of warrants at $62.50 per share, 800
shares of common stock that may be acquired upon the exercise of
warrants at $12.50 per share, 4,000 shares of common stock that may
be acquired upon the exercise of warrants at $1.25 per share, and
52,500 shares of common stock that may be acquired upon the
exercise of warrants at $0.09 per share.
(14)
Includes 2,800
shares or the Company’s common stock that may be acquired
upon the exercise of warrants at $62.50 per share, 1,400 per share,
16,000 shares of common stock that may be acquired upon the
exercise of warrants at $1.25 per share, and 500,000 shares of
common stock that may be acquired upon the exercise of warrants at
$0.09 per share.
(15)
Consists of 8,000
shares of common stock that may be acquired upon the exercise of
warrants at $1.25 per share, and 52,500 shares of common stock that
may be acquired upon the exercise of warrants at $0.09 per
share.
Other
than as set forth above, the issuer is not aware of any person who
owns of record, or is known to own beneficially, five percent (5%)
or more of the outstanding securities of any class of the
issuer.
There
are no current arrangements which will result in a change in
control.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Joint Venture
On
September 13, 2016, we entered into an operating agreement to form
a pain management joint venture company with Advanced Technologies
Solutions (ATS), a company owned by Ronald T. La Borde, a member of
our Board of Directors. The joint venture company, Premier
Biomedical Pain Management Solutions, LLC, a Nevada limited
liability company (PBPMS), was formed to develop and market natural
and cannabis-based generalized, neuropathic, and localized pain
relief treatment products. We owned 50% of PBPMS and ATS owned the
other 50%, with 89% of the profits allocated to us and the
remaining 11% of profits allocated to ATS.
The
PBPMS operating agreement called for ATS to enter into a licensing
agreement with PBPMS. Upon entering into the license agreement, Mr.
La Borde was to receive 5,000 warrants to purchase our common stock
at an exercise price of $12.50 per share.
However,
the license agreement was never entered into, the joint venture was
terminated, and PBPMS was dissolved on September 19,
2017.
License Agreements
On May
12, 2010, we entered into two separate license agreements. One
license agreement was entered into with Altman Enterprises, LLC,
wherein we obtained certain exclusive rights in (i) proprietary
technology that is the subject of one pending PCT patent
application relating to the treatment of auto-immune diseases, and
(ii) the Feldetrex®
trademark. The other license agreement was entered into with Marv
Enterprises, LLC, wherein we obtained certain exclusive rights in
proprietary technology that is the subject of two PCT patent
applications relating to the treatment of blood borne carcinomas
and sequential extracorporeal treatment of blood. Authority to
execute the license agreements on behalf of Altman and Marv is
vested in Dr. Mitchell S. Felder, the Chairman of our Board of
Directors. Because the licensors are controlled by one of our
directors, there may exist a conflict of interest in decisions made
by the Company with respect to the licenses.
As
consideration for the two licenses, we agreed to (i) pay a royalty
of five percent (5%) of any sales of products using the technology,
with no minimum royalty, and (ii) reimburse the licensor for any
costs already incurred in pursuing its proprietary rights in the
licensed technology and pay any costs incurred for maintaining or
obtaining the licensors’ proprietary rights in the licensed
technology in the U.S. and in extending the intellectual property
to other countries around the world. Licensor shall have sole
discretion to select other countries into which exclusive rights in
the licensed technology may be pursued, and if we decline to pay
those expenses, then licensor may pay said expenses and our
licensed rights in those countries will revert to the
licensor.
As of
December 31, 2018, we have not sold any products using the licensed
technology and thus have not paid any license fees. We have,
however, reimbursed expenses associated with the technology we have
licensed, and owe them an additional $46,016.
Stock Issuances
Preferred Stock
On
January 2, 2016, two of our officers and directors, William A.
Hartman and Mitchell Felder, each exercised warrants to acquire one
million (1,000,000) shares of Series A Convertible Preferred Stock
each. Each share of Series A Convertible Preferred Stock is
convertible, at the election of the holder thereof, into 0.004 of a
share of our common stock, and has one hundred (100) votes per
share. We issued the warrants on June 21, 2010 and they had an
exercise price of $0.001 per share.
The
Preferred Stock also contains protective provisions as
follows:
The
Company may not take any of the following actions without the
approval of a majority of the holders of the outstanding Series A
Convertible Preferred Stock: (i) effect a sale of all or
substantially all of the Company’s assets or which results in
the holders of the Company’s capital stock prior to the
transaction owning less than fifty percent (50%) of the voting
power of the Company’s capital stock after the transaction,
(ii) alter or change the rights, preferences, or privileges of
the Series A Convertible Preferred Stock, (iii) increase or
decrease the number of authorized shares of Series A Convertible
Preferred Stock, (iv) authorize the issuance of securities having a
preference over or on par with the Series A Convertible Preferred
Stock, or (v) effectuate a forward or reverse stock split or
dividend of the Company’s common stock.
Warrant Exercise
On
November 22, 2017, we issued 28,000 shares of common stock,
restricted in accordance with Rule 144, to William Hartman, an
officer and director of the Company, upon the exercise of warrants
at $0.63 per share.
On
December 20, 2016, we issued 24,000 shares of common stock,
restricted in accordance with Rule 144, to each of William Hartman
and Mitchell Felder, officers and directors of the Company, upon
the exercise of warrants at $0.63 per share.
On
August 19, 2016, we issued 16,000 shares of common stock,
restricted in accordance with Rule 144, to each of William Hartman
and Mitchell Felder, officers and directors of the Company, upon
the exercise of warrants at $0.63 per share.
DISCLOSURE OF COMMISSION POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Article
9 of our Articles of Incorporation provides that, the personal
liability of the directors of the corporation is hereby eliminated
to the fullest extent permitted by paragraph 1 of Section 78.037 of
the General Corporation Law of the State of Nevada, as the same may
be amended and supplemented. Paragraph 1 of Section 78.037 states
that the articles of incorporation of a Nevada corporation may
contain any provision, not contrary to the laws of the State of
Nevada, for the management of the business and for the conduct of
the affairs of the corporation.
Article
10 of our Articles of Incorporation provides that, the corporation
shall, to the fullest extent permitted by Section 78.751 of the
General Corporation Law of the State of Nevada, as the same may be
amended and supplemented, indemnify any and all persons whom it
shall have power to indemnify under said section from and against
any and all expenses, liabilities, or other matters referred to in
or covered by said section. Section 78.751 states that the articles
of incorporation of a Nevada corporation may provide that the
expenses of officers and directors incurred in defending a civil or
criminal action, suit or proceeding must be paid by the corporation
as they are incurred and in advance of the final disposition. It
further states that indemnification does not exclude any other
rights that an officer or director may have pursuant to the
articles, bylaws, shareholders agreement or otherwise, and that it
continues for a person who has ceased to be a director, officer, or
employee of the company.
Article
V of our Bylaws further addresses indemnification, including
procedures for indemnification claims. Indemnification applies to
any person that is made a party to, or threatened to be made a
party to, any action, suit or proceeding, whether civil, criminal,
administrative or investigative, by reason of the fact that he or
she was an officer or director of the company.
Insofar
as indemnification for liabilities arising under the Securities Act
of 1933 (the “Act”) may be permitted to
directors, officers and controlling persons of the registrant
pursuant to the foregoing provisions, or otherwise, the registrant
has been advised that in the opinion of the Securities and Exchange
Commission such indemnification is against public policy as
expressed in the Act and is, therefore, unenforceable.
WHERE YOU CAN FIND MORE INFORMATION
We have
filed with the Securities and Exchange Commission a registration
statement on Form S-1, together with all amendments and exhibits
thereto, under the Securities Act of 1933 with respect to the
common stock offered hereby. This Prospectus, which constitutes a
part of the registration statement, does not contain all of the
information set forth in the registration statement and the
exhibits and schedules thereto. You should refer to the
registration statement and its exhibits and schedules for further
information. Statements contained in this Prospectus as to the
contents of any contract or other document referred to are not
necessarily complete and in each instance reference is made to the
copy of such contract or other document filed as an exhibit to the
registration statement, each such statement being qualified in all
respects by such reference.
Copies
of documents we file with the Commission, including this
prospectus, the registration of which it is a part and the related
exhibits, may be read and copies at the Commission’s Public
Reference Room at 100 F Street, NE, Washington, DC 20549. Please
call the SEC at 1-800-SEC-0330 for further information on the
public reference room. Our filings with the Commission are also
available through the Commission’s website at the following
address: http://www.sec.gov.
We are
subject to the information and periodic reporting requirements of
the Securities Exchange Act of 1934, and, in accordance therewith,
file periodic reports, proxy statements and other information with
the Commission. Such periodic reports, proxy statements and other
information are available for inspection and copying at the Public
Reference Room and website of the SEC referred to above. We also
furnish our shareholders with annual reports containing our
financial statements audited by an independent registered public
accounting firm and quarterly reports containing our unaudited
financial information. We maintain a website at
www.premierbiomedical.com. You may access our annual report on Form
10-K, quarterly reports on Form 10-Q, current reports on Form 8-K,
and amendments to those reports, filed or furnished pursuant to
section 13(a) or 15(d) of the Exchange Act with the Commission free
of charge at our website as soon as reasonably practicable after
this material is electronically filed with, or furnished to, the
Commission. The reference to our website or web address does not
constitute incorporation by reference of the information contained
at that site.
INCORPORATION BY REFERENCE
We
“incorporate by reference” information from other
documents that we file with the SEC into this prospectus, which
means that we disclose important information to you by referring
you to those documents. The information incorporated by reference
is deemed to be part of this prospectus except for any information
that is superseded by information included directly in this
prospectus, and the information that we file later with the SEC
will automatically supersede this information. Any statement
contained in this prospectus or any prospectus supplement or a
document incorporated by reference in this prospectus or in any
prospectus supplement will be deemed to be modified or superseded
for purposes of this prospectus to the extent that a statement
contained in this prospectus or in any other subsequently filed
document that is incorporated by reference in this prospectus
modifies or superseded the statement. Any statement so modified or
superseded will not be deemed, except as so modified or superseded,
to constitute a part of this prospectus. You should not assume that
the information in this prospectus is current as of the date other
than the date on the cover page of this prospectus.
We are
incorporating by reference into this prospectus any additional
documents that we may file with the SEC pursuant to Sections 13(a),
13(c), 14 or 15(d) of the Exchange Act on or after the effective
date of the registration statement and prior to the termination of
the offering.
You may
request a copy of any document incorporated by reference in this
prospectus and any exhibit specifically incorporated by reference
in those documents, at no cost, by writing or telephoning us at the
following address or phone number:
Premier
Biomedical, Inc.
P.O.
Box 25
Jackson
Center, PA 16133
Attn:
Investor Relations
(724)
633-7033
EXPERTS
The
audited financial statements of Premier Biomedical, Inc. as of
December 31, 2018 and 2017 appearing in this prospectus which is
part of a registration statement have been so included in reliance
on the report of M&K CPAS, PLLC, given on the authority of such
firm as experts in accounting and auditing.
INDEX TO FINANCIAL STATEMENTS
For the
Three and Nine Months ended September 30, 2019 and
2018
|
Condensed
Consolidated Balance Sheets as of September 30, 2019 and December
31, 2018 (Unaudited)
|
F-1
|
|
|
|
|
Condensed
Consolidated Statements of Operations for the three and nine months
ended September 30, 2019 and 2018 (Unaudited)
|
F-2
|
|
|
|
|
Condensed
Consolidated Statement of Stockholders’ Equity
(Deficit) for the nine months ended September 30, 2019 and 2018
(Unaudited)
|
F-3
|
|
|
|
|
Condensed
Consolidated Statements of Cash Flows for the nine months
ended September 30, 2019 and 2018 (Unaudited)
|
F-4
|
|
|
|
|
Notes
to Condensed
Consolidated Financial Statements
|
F-5 to
F-20
|
For the
Years ended December 31, 2018 and 2017
|
Report
of Independent Registered Public Accounting Firm
|
F-21
|
|
|
|
|
Balance
Sheets as of December 31, 2018 and 2017
(Audited)
|
F-22
|
|
|
|
|
Statements of
Operations for the years ended December 31, 2018 and 2017
(Audited)
|
F-23
|
|
|
|
|
Statement of
Stockholders’ Equity (Deficit) for the years ended December
31, 2018 and 2017 (Audited)
|
F-24
|
|
|
|
|
Statements of Cash
Flows for the years ended December 31, 2018 and 2017
(Audited)
|
F-25
|
|
|
|
|
Notes
to Financial Statements
|
F-26 to
F-44
|
PREMIER
BIOMEDICAL, INC.
CONDENSED
CONSOLIDATED BALANCE SHEETS
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
Current
assets:
|
|
|
|
Cash
|
$117,209
|
$86,827
|
|
Accounts
receivable
|
3,387
|
3,092
|
|
Inventory
|
20,516
|
25,985
|
|
Other current
assets
|
36,470
|
43,883
|
|
Total current
assets
|
177,582
|
159,787
|
|
|
|
|
|
Property and
equipment, net
|
7,918
|
5,203
|
|
|
|
|
|
Total
assets
|
$185,500
|
$164,990
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS' EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
Accounts
payable
|
$192,543
|
$264,398
|
|
Accounts payable,
related parties
|
31,081
|
25,944
|
|
Accrued
interest
|
49,148
|
22,099
|
|
Convertible notes
payable, net of discounts of $278,228 and $-0- at September 30,
2019
|
|
|
|
and December 31,
2018, respectively, including $139,614 currently in
default
|
192,286
|
309,637
|
|
Derivative
liabilities
|
1,838,652
|
1,690,304
|
|
Total current
liabilities
|
2,303,710
|
2,312,382
|
|
|
|
|
|
Total
liabilities
|
2,303,710
|
2,312,382
|
|
|
|
|
|
Commitments and
contingencies
|
-
|
-
|
|
|
|
|
|
Stockholders'
equity (deficit):
|
|
|
|
Series A
convertible preferred stock, $0.001 par value, 10,000,000 shares
authorized, 2,000,000 shares designated, issued
and outstanding at September 30, 2019 and December 31, 2018,
respectively
|
2,000
|
2,000
|
|
Series B
convertible preferred stock, $0.001 par value, 1,000,000 shares
designated, 133,780 shares issued and
outstanding at September 30, 2019 and December 31, 2018,
respectively
|
134
|
150
|
|
Common stock,
$0.00001 par value, 1,000,000,000 shares authorized, 49,216,810 and
5,652,410 shares issued and
outstanding at September 30, 2019 and December 31, 2018,
respectively
|
492
|
57
|
|
Additional paid in
capital
|
14,947,033
|
14,572,754
|
|
Subscriptions
payable, consisting of 276,960 shares at December 31,
2018
|
-
|
5,345
|
|
Accumulated
deficit
|
(17,067,869)
|
(16,727,698)
|
|
Total stockholders'
equity (deficit)
|
(2,118,210)
|
(2,147,392)
|
|
|
|
|
|
Total liabilities
and stockholders' equity (deficit)
|
$185,500
|
$164,990
|
See accompanying
notes to financial statements.
PREMIER
BIOMEDICAL, INC.
CONDENSED
CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
$3,465
|
$8,225
|
$12,975
|
$30,709
|
|
Cost of goods
sold
|
1,653
|
4,373
|
5,470
|
20,577
|
|
Gross
profit
|
1,812
|
3,852
|
7,505
|
10,132
|
|
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
|
|
General and
administrative
|
36,757
|
62,572
|
138,589
|
139,881
|
|
Professional
fees
|
29,609
|
22,613
|
88,604
|
100,953
|
|
Total operating
expenses
|
66,366
|
85,185
|
227,193
|
240,834
|
|
|
|
|
|
|
|
Net operating
loss
|
(64,554)
|
(81,333)
|
(219,688)
|
(230,702)
|
|
|
|
|
|
|
|
Other income
(expense):
|
|
|
|
|
|
Interest
expense
|
(56,002)
|
(184,624)
|
(101,793)
|
(322,323)
|
|
Change in
derivative liabilities
|
(61,078)
|
(97,578)
|
(18,690)
|
655,808
|
|
Total other income
(expense)
|
(117,080)
|
(282,202)
|
(120,483)
|
333,485
|
|
|
|
|
|
|
|
Net income
(loss)
|
$(181,634)
|
$(363,535)
|
$(340,171)
|
$102,783
|
|
|
|
|
|
|
|
Weighted average
number of common shares outstanding - basic
|
26,817,415
|
3,306,069
|
14,837,666
|
3,058,442
|
|
Weighted average
number of common shares outstanding - fully diluted
|
26,817,415
|
3,306,069
|
14,837,666
|
3,070,392
|
|
|
|
|
|
|
|
Net income (loss)
per share - basic
|
$(0.01)
|
$(0.11)
|
$(0.02)
|
$0.03
|
|
Net income (loss)
per share - fully diluted
|
$(0.01)
|
$(0.11)
|
$(0.02)
|
$0.03
|
See accompanying
notes to financial statements.
PREMIER
BIOMEDICAL, INC.
STATEMENT
OF STOCKHOLDERS' EQUITY (DEFICIT)
|
|
|
Series
B Convertible
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2017
|
2,000,000
|
$2,000
|
-
|
$-
|
2,551,443
|
$26
|
$13,442,255
|
$273,805
|
$(16,328,812)
|
$(2,610,726)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on subsctiptions payable
|
-
|
-
|
-
|
-
|
254,703
|
2
|
273,803
|
(273,805)
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on debt conversions
|
-
|
-
|
-
|
-
|
183,161
|
2
|
64,998
|
-
|
-
|
65,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments
to derivative liability due to debt conversions
|
-
|
-
|
-
|
-
|
-
|
-
|
52,270
|
-
|
-
|
52,270
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
income for the three months ended March 31, 2018
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
566,324
|
566,324
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
March 31, 2018
|
2,000,000
|
$2,000
|
-
|
$-
|
2,989,307
|
$30
|
$13,833,326
|
$-
|
$(15,762,488)
|
$(1,927,132)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the three months ended June 30, 2018
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(100,006)
|
(100,006)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
June 30, 2018
|
2,000,000
|
$2,000
|
-
|
$-
|
2,989,307
|
$30
|
$13,833,326
|
$-
|
$(15,862,494)
|
$(2,027,138)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on debt conversions
|
-
|
-
|
-
|
-
|
784,542
|
8
|
58,012
|
12,500
|
-
|
70,520
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments
to derivative liability due to debt conversions
|
-
|
-
|
-
|
-
|
-
|
-
|
76,401
|
-
|
-
|
76,401
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the three months ended Septmeber 30, 2018
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(363,535)
|
(363,535)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
September 30, 2018
|
2,000,000
|
$2,000
|
-
|
$-
|
3,773,849
|
$38
|
$13,967,739
|
$12,500
|
$(16,226,029)
|
$(2,243,752)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on subsctiptions payable
|
-
|
-
|
-
|
-
|
172,176
|
2
|
12,498
|
(12,500)
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series
B convertible preferred stock sold for cash
|
-
|
-
|
150,000
|
150
|
-
|
-
|
149,850
|
-
|
-
|
150,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on debt conversions
|
-
|
-
|
-
|
-
|
1,694,385
|
17
|
74,737
|
5,345
|
-
|
80,099
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise
of warrants at $0.00001 per share, related parties
|
-
|
-
|
-
|
-
|
12,000
|
-
|
30
|
-
|
-
|
30
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
issued for services, related parties
|
-
|
-
|
-
|
-
|
-
|
-
|
272,585
|
-
|
-
|
272,585
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants
issued for services
|
-
|
-
|
-
|
-
|
-
|
-
|
24,359
|
-
|
-
|
24,359
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments
to derivative liability due to debt conversions
|
-
|
-
|
-
|
-
|
-
|
-
|
70,956
|
-
|
-
|
70,956
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the three months ended December 31, 2018
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(501,669)
|
(501,669)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2018
|
2,000,000
|
$2,000
|
150,000
|
$150
|
5,652,410
|
$57
|
$14,572,754
|
$5,345
|
$(16,727,698)
|
$(2,147,392)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on subscriptions payable
|
-
|
-
|
-
|
-
|
276,960
|
3
|
5,342
|
(5,345)
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on debt conversions
|
-
|
-
|
-
|
-
|
2,578,585
|
25
|
50,765
|
6,500
|
-
|
57,290
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments
to derivative liability due to debt conversions
|
-
|
-
|
-
|
-
|
-
|
-
|
48,807
|
-
|
-
|
48,807
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the three months ended March 31, 2019
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
68,511
|
68,511
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
March 31, 2019 (Unaudited)
|
2,000,000
|
$2,000
|
150,000
|
$150
|
8,507,955
|
$85
|
$14,677,668
|
$6,500
|
$(16,659,187)
|
$(1,972,784)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on debt conversions
|
-
|
-
|
-
|
-
|
5,024,475
|
50
|
42,658
|
(6,500)
|
-
|
36,208
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments
to derivative liability due to debt conversions
|
-
|
-
|
-
|
-
|
-
|
-
|
20,475
|
-
|
-
|
20,475
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the three months ended June 30, 2019
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(227,048)
|
(227,048)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
June 30, 2019 (Unaudited)
|
2,000,000
|
$2,000
|
150,000
|
$150
|
13,532,430
|
$135
|
$14,740,801
|
$-
|
$(16,886,235)
|
$(2,143,149)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred
stock issued on conversions
|
-
|
-
|
(16,220)
|
(16)
|
4,689,556
|
47
|
(31)
|
-
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common
stock issued on debt conversions
|
-
|
-
|
-
|
-
|
30,994,824
|
310
|
97,319
|
-
|
-
|
97,629
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments
to derivative liability due to debt conversions
|
-
|
-
|
-
|
-
|
-
|
-
|
108,944
|
-
|
-
|
108,944
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
loss for the three months ended September 30, 2019
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(181,634)
|
(181,634)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
September 30, 2019 (Unaudited)
|
2,000,000
|
$2,000
|
133,780
|
$134
|
49,216,810
|
$492
|
$14,947,033
|
$-
|
$(17,067,869)
|
$(2,118,210)
|
See
accompanying notes to financial statements.
PREMIER
BIOMEDICAL, INC.
CONDENSED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
CASH
FLOWS FROM OPERATING ACTIVITIES
|
|
|
|
Net income
(loss)
|
$(340,171)
|
$102,783
|
|
Adjustments to
reconcile net income (loss)
|
|
|
|
to net cash used in
operating activities:
|
|
|
|
Depreciation
|
2,135
|
1,749
|
|
Change in fair
market value of derivative liabilities
|
18,690
|
(655,808)
|
|
Amortization of
debt discounts
|
59,556
|
306,442
|
|
Decrease (increase)
in assets:
|
|
|
|
Accounts
receivable
|
(295)
|
(1,654)
|
|
Inventory
|
5,469
|
(34,392)
|
|
Other current
assets
|
7,413
|
(3,242)
|
|
Increase (decrease)
in liabilities:
|
|
|
|
Accounts
payable
|
(71,855)
|
(39,787)
|
|
Accounts payable,
related parties
|
5,137
|
(10,443)
|
|
Accrued
interest
|
40,753
|
15,881
|
|
Net cash used in
operating activities
|
(273,168)
|
(318,471)
|
|
|
|
|
|
CASH
FLOWS FROM INVESTING ACTIVITIES
|
|
|
|
Purchases of
property and equipment
|
(4,850)
|
(2,029)
|
|
Net cash used in
investing activities
|
(4,850)
|
(2,029)
|
|
|
|
|
|
CASH
FLOWS FROM FINANCING ACTIVITIES
|
|
|
|
Proceeds from
convertible notes payable
|
308,400
|
300,000
|
|
Net cash provided
by financing activities
|
308,400
|
300,000
|
|
|
|
|
|
NET CHANGE IN
CASH
|
30,382
|
(20,500)
|
|
CASH AT BEGINNING
OF PERIOD
|
86,827
|
83,704
|
|
|
|
|
|
CASH AT END OF
PERIOD
|
$117,209
|
$63,204
|
|
|
|
|
|
SUPPLEMENTAL
INFORMATION:
|
|
|
|
Interest
paid
|
$1,484
|
$-
|
|
Income taxes
paid
|
$-
|
$-
|
|
|
|
|
|
NON-CASH INVESTING
AND FINANCING ACTIVITIES:
|
|
|
|
Value of preferred
stock converted to common stock
|
$44,913
|
$-
|
|
Value of debt
discounts
|
$304,311
|
$300,000
|
|
Value of derivative
adjustment due to debt conversions
|
$178,226
|
$128,671
|
|
Value of shares
issued for conversion of debt
|
$191,127
|
$135,520
|
See accompanying
notes to financial statements.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
Note
1 – Basis of Presentation and Significant Accounting
Policies
Basis of Presentation
The accompanying
unaudited, condensed consolidated financial statements of Premier
Biomedical, Inc. (“the Company”) have been prepared
pursuant to rules and regulations of the Securities and Exchange
Commission (“SEC”) and, therefore, do not include all
information and footnote disclosures normally included in audited
financial statements. However, these statements reflect all
adjustments, consisting of normal recurring adjustments, which in
the opinion of management are necessary for fair presentation of
the information contained therein. It is suggested that these
statements be read in conjunction with the financial statements
included in the Company’s Annual Report on Form 10-K for
the year ended December 31, 2018.
Use of Estimates
The preparation of
financial statements in conformity with accounting principles
generally accepted in the United States requires management to make
estimates and assumptions that affect the reported amounts of
assets and liabilities, and the disclosure of contingent assets and
liabilities at the date of the financial statements, and the
reported amounts of revenues and expenses during the reporting
period. Actual results could differ from those
estimates.
Cash and Cash Equivalents
We maintain cash
balances in non-interest-bearing accounts, which do not currently
exceed federally insured limits. For the purpose of the statements
of cash flows, all highly liquid investments with an original
maturity of three months or less are considered to be cash
equivalents.
Patent Rights and Applications
Patent rights and
applications costs include the acquisition costs and costs incurred
for the filing of patents. Patent rights and applications are
amortized on a straight-line basis over the legal life of the
patent rights beginning at the time the patents are approved.
Patent costs for unsuccessful patent applications are expensed when
the application is terminated.
Fair Value of Financial Instruments
Under FASB ASC
820-10-05, the Financial Accounting Standards Board establishes a
framework for measuring fair value in generally accepted accounting
principles and expands disclosures about fair value measurements.
This Statement reaffirms that fair value is the relevant
measurement attribute. The adoption of this standard did not have a
material effect on the Company’s financial statements as
reflected herein. The carrying amounts of cash, prepaid expenses
and accrued expenses reported on the balance sheet are estimated by
management to approximate fair value primarily due to the
short-term nature of the instruments.
Basic and Diluted Loss Per Share
Basic earnings per
share (“EPS”) are computed by dividing net income (the
numerator) by the weighted average number of common shares
outstanding for the period (the denominator). Diluted EPS is
computed by dividing net income by the weighted average number of
common shares and potential common shares outstanding (if dilutive)
during each period. Potential common shares include stock options,
warrants and restricted stock. The number of potential common
shares outstanding relating to stock options, warrants and
restricted stock is computed using the treasury stock
method.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
The reconciliation
of the denominators used to calculate basic EPS and diluted EPS for
the nine months ended September 30, 2019 and 2018 are as
follows:
|
|
For the Nine Months
Ended
|
|
|
|
|
|
|
|
|
Weighted average
common shares outstanding – basic
|
14,837,666
|
3,058,442
|
|
Plus: Potentially
dilutive common shares:
|
|
|
|
Warrants
|
-
|
11,950
|
|
Weighted average
common shares outstanding – diluted
|
14,837,666
|
3,070,392
|
For
the nine months ended September 30, 2019, potential dilutive
securities had an anti-dilutive effect and were not included in the
calculation of diluted net loss per common share. Warrants excluded
from the calculation of diluted EPS because their effect was
anti-dilutive were 3,898,000 and 245,760 as of
September 30, 2019 and 2018, respectively.
Stock-Based Compensation
Under FASB ASC
718-10-30-2, all share-based payments to employees, including
grants of employee stock options, to be recognized in the income
statement based on their fair values. Pro forma disclosure is no
longer an alternative. The Company had no stock-based compensation
issuances during the nine months ended September 30, 2019
and 2018.
Revenue Recognition
On
January 1, 2018, we adopted Accounting Standards Update No.
2014-09, Revenue from Contracts with Customers (Topic 606), which
supersedes the revenue recognition requirements in Accounting
Standards Codification (ASC) Topic 605, Revenue Recognition (Topic
605). Results for reporting periods beginning after January 1, 2018
are presented under Topic 606. The impact of adopting the new
revenue standard was not material to our financial statements and
there was no adjustment to beginning retained earnings on January
1, 2018.
Under
Topic 606, revenue is recognized when control of the promised goods
or services is transferred to our customers, in an amount that
reflects the consideration we expect to be entitled to in exchange
for those goods or services.
We
determine revenue recognition through the following
steps:
●
identification of
the contract, or contracts, with a customer;
●
identification of
the performance obligations in the contract;
●
determination of
the transaction price;
●
allocation of the
transaction price to the performance obligations in the contract;
and
●
recognition of
revenue when, or as, we satisfy a performance
obligation.
Sales
are recorded when the earnings process is complete or substantially
complete, and the revenue is measurable and collectability is
reasonably assured, which is typically when products are shipped.
Provisions for discounts and rebates to customers, estimated
returns and allowances, and other adjustments are provided for in
the same period the related sales are recorded. The Company defers
any revenue from sales in which payment has been received, but the
earnings process has not been completed. Sales commenced on July 5,
2017 with the termination of our joint venture.
Advertising and Promotion
All costs
associated with advertising and promoting products are expensed as
incurred. These expenses were $35,696 and $50,127 for the nine
months ended September 30, 2019 and 2018,
respectively.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
Income Taxes
Deferred tax assets
and liabilities are recognized for the future tax consequences
attributable to differences between the financial statement
carrying amounts of existing assets and liabilities and their
respective tax bases. Deferred tax assets and liabilities are
measured using enacted tax rates expected to apply to taxable
income in the years in which those temporary differences are
expected to be recovered or settled. A valuation allowance is
provided for significant deferred tax assets when it is more likely
than not, that such asset will not be recovered through future
operations.
Uncertain Tax Positions
In accordance with
ASC 740, “Income Taxes” (“ASC 740”), the
Company recognizes the tax benefit from an uncertain tax position
only if it is more likely than not that the tax position will be
capable of withstanding examination by the taxing authorities based
on the technical merits of the position. These standards prescribe
a recognition threshold and measurement attribute for the financial
statement recognition and measurement of a tax position taken or
expected to be taken in a tax return. These standards also provide
guidance on de-recognition, classification, interest and penalties,
accounting in interim periods, disclosure, and
transition.
Various taxing
authorities periodically audit the Company’s income tax
returns. These audits include questions regarding the
Company’s tax filing positions, including the timing and
amount of deductions and the allocation of income to various tax
jurisdictions. In evaluating the exposures connected with these
various tax filing positions, including state and local taxes, the
Company records allowances for probable exposures. A number of
years may elapse before a particular matter, for which an allowance
has been established, is audited and fully resolved. The Company
has not yet undergone an examination by any taxing
authorities.
The assessment of
the Company’s tax position relies on the judgment of
management to estimate the exposures associated with the
Company’s various filing positions.
Recent Accounting Pronouncements
In August 2018, the
Financial Accounting Standards Board (“FASB”) issued
Accounting Standards Update (“ASU”) 2018-13,
Fair Measurement (Topic 820):
Disclosure Framework – Changes to the Disclosure Requirements
for Fair Value Measurement, which modify the disclosure
requirements of Topic 820. The new guidance is effective for all
entities for annual periods, and interim periods within those
annual periods, beginning after December 15, 2019, with early
adoption permitted. The Company does not expect the adoption of
this ASU to have a material impact on its consolidated financial
statements.
In July 2018, the
FASB issued ASU No. 2018-10, Codification Improvements to Topic 842,
Leases. The amendments in ASU 2018-10 provide additional
clarification and implementation guidance on certain aspects of the
previously issued ASU No. 2016-02, Leases (Topic 842) (“ASU
2016-02”) and have the same effective and transition
requirements as ASU 2016-02. Upon the effective date, ASU 2018-10
will supersede the current lease guidance in ASC Topic 840, Leases.
Under the new guidance, lessees will be required to recognize for
all leases, with the exception of short-term leases, a lease
liability, which is a lessee’s obligation to make lease
payments arising from a lease, measured on a discounted basis.
Concurrently, lessees will be required to recognize a right-of-use
asset, which is an asset that represents the lessee’s right
to use, or control the use of, a specified asset for the lease
term. ASU 2018-10 is effective for private companies and emerging
growth public companies for interim and annual reporting periods
beginning after December 15, 2019, with early adoption permitted.
The guidance is required to be applied using a modified
retrospective transition approach for leases existing at, or
entered into after, the beginning of the earliest comparative
periods presented in the financial statements. The Company adopted
this guidance effective January 1, 2019, and the standard did not
have a material impact on the Company’s combined financial
statements and related disclosures.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
In June 2018, the
FASB issued ASU 2018-07, Compensation-Stock Compensation (Topic 718):
Improvements to Nonemployee Share-Based Payment Accounting,
which expands the scope of Topic 718 to include share-based payment
transactions for acquiring goods and services from nonemployees. An
entity should apply the requirements of Topic 718 to nonemployee
awards except for specific guidance on inputs to an option pricing
model and the attribution of cost (that is, the period of time over
which share-based payment awards vest and the pattern of cost
recognition over that period). The new guidance is effective for
all entities for annual periods, and interim periods within those
annual periods, beginning after December 15, 2017, with early
adoption permitted. The adoption of this ASU has not had a material
impact on its consolidated financial statements.
In March 2018, the
FASB issued ASU No. 2018-05, Income Taxes (Topic 740) - Amendments to SEC
Paragraphs Pursuant to SEC Staff Accounting Bulletin No.
118. The amendment provides guidance on accounting for the
impact of the Tax Cuts and Jobs Act (the “Tax Act”) and
allows entities to complete the accounting under ASC 740 within a
one-year measurement period from the Tax Act enactment date. This
standard is effective upon issuance. The Tax Act has several
significant changes that impact all taxpayers, including a
transition tax, which is a one-time tax charge on accumulated,
undistributed foreign earnings. The calculation of accumulated
foreign earnings requires an analysis of each foreign
entity’s financial results going back to 1986. The adoption
of this ASU has not had a material impact on its consolidated
financial statements.
In February 2018, the FASB issued ASU No.
2018-02, Reclassification of Certain
Tax Effects from Accumulated Other Comprehensive
Income. The guidance permits
entities to reclassify tax effects stranded in Accumulated Other
Comprehensive Income as a result of tax reform to retained
earnings. This new guidance is effective for annual and interim
periods in fiscal years beginning after December 15, 2018. Early
adoption is permitted in annual and interim periods and can be
applied retrospectively or in the period of adoption. The
adoption of this ASU has not had a material impact on its
consolidated financial statements.
Effective January
1, 2018, the Company adopted ASC 606 — Revenue from Contracts
with Customers. Under ASC 606, the Company recognizes revenue from
the commercial sales of products, licensing agreements and
contracts to perform pilot studies by applying the following steps:
(1) identify the contract with a customer; (2) identify the
performance obligations in the contract; (3) determine the
transaction price; (4) allocate the transaction price to each
performance obligation in the contract; and (5) recognize revenue
when each performance obligation is satisfied. For the comparative
periods, revenue has not been adjusted and continues to be reported
under ASC 605 — Revenue Recognition. Under ASC 605, revenue
is recognized when the following criteria are met: (1) persuasive
evidence of an arrangement exists; (2) the performance of service
has been rendered to a customer or delivery has occurred; (3) the
amount of fee to be paid by a customer is fixed and determinable;
and (4) the collectability of the fee is reasonably assured.
There was no impact on the
Company’s financial statements as a result of adopting Topic
606 for the years ended December 31, 2018 and
2017.
In February 2016, the FASB issued ASU
2016-02, Leases (Topic
842). ASU 2016-02 requires
lessees to recognize assets and liabilities for most leases. ASU
2016-02 is effective for public entity financial statements for
annual periods beginning after December 15, 2018, and interim
periods within those annual periods. Early adoption is permitted,
including adoption in an interim period. ASU 2016-02 was further
clarified and amended within ASU 2018-01, ASU 2018-10, ASU 2018-11
and ASU 2018-20 which included provisions that would provide us
with the option to adopt the provisions of the new guidance using a
modified retrospective transition approach, without adjusting the
comparative periods presented. We adopted the new standard on
January 1, 2019 and used the effective date as our date of initial
application under the modified retrospective approach. We elected
the short-term lease recognition exemption for all of our leases
that qualify. This means, for those leases we will not recognize
right-of-use (RoU) assets or lease liabilities. The implementation
of this new standard has no impact on our financial
statements.
No other new
accounting pronouncements, issued or effective during the nine
months ended September 30, 2019, have had or are expected to
have a significant impact on the Company’s financial
statements.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
Note
2 – Going Concern
As shown in the
accompanying financial statements, the Company has minimal
revenues, incurred net losses from operations resulting in an
accumulated deficit of $17,067,869, and had negative working
capital of ($2,126,128) at September 30, 2019. These factors
raise substantial doubt about the Company’s ability to
continue as a going concern. The Company is currently seeking
additional sources of capital to fund short term operations. The
Company, however, is dependent upon its ability to secure equity
and/or debt financing and there are no assurances that the Company
will be successful; therefore, without sufficient financing it
would be unlikely for the Company to continue as a going
concern.
The financial
statements do not include any adjustments that might result from
the outcome of any uncertainty as to the Company’s ability to
continue as a going concern. The financial statements also do not
include any adjustments relating to the recoverability and
classification of recorded asset amounts, or amounts and
classifications of liabilities that might be necessary should the
Company be unable to continue as a going concern.
Note
3 – Related Parties
Accounts Payable
The Company owed
$30,006 and $24,116 as of September 30, 2019 and December 31,
2018, respectively, to entities owned by the Chairman of the Board
of Directors. The amounts are related to patent costs and
reimbursable expenses paid by the Chairman on behalf of the
Company.
The Company owed
$753 as of December 31, 2018 to the Company’s CEO for
reimbursable expenses.
The Company owed
$1,075 as of September 30, 2019 and December 31, 2018 amongst
members of the Company’s Board of Directors for reimbursable
expenses.
Note
4 – Fair Value of Financial Instruments
Under FASB ASC
820-10-5, fair value is defined as the price that would be received
to sell an asset or paid to transfer a liability in an orderly
transaction between market participants at the measurement date (an
exit price). The standard outlines a valuation framework and
creates a fair value hierarchy in order to increase the consistency
and comparability of fair value measurements and the related
disclosures. Under GAAP, certain assets and liabilities must be
measured at fair value, and FASB ASC 820-10-50 details the
disclosures that are required for items measured at fair
value.
The Company has
certain financial instruments that must be measured under the new
fair value standard. The Company’s financial assets and
liabilities are measured using inputs from the three levels of the
fair value hierarchy. The three levels are as follows:
Level 1 - Inputs
are unadjusted quoted prices in active markets for identical assets
or liabilities that the Company has the ability to access at the
measurement date.
Level 2 - Inputs
include quoted prices for similar assets and liabilities in active
markets, quoted prices for identical or similar assets or
liabilities in markets that are not active, inputs other than
quoted prices that are observable for the asset or liability (e.g.,
interest rates, yield curves, etc.), and inputs that are derived
principally from or corroborated by observable market data by
correlation or other means (market corroborated
inputs).
Level 3 -
Unobservable inputs that reflect our assumptions about the
assumptions that market participants would use in pricing the asset
or liability.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
The following
schedule summarizes the valuation of financial instruments at fair
value on a recurring basis in the balance sheets as of
September 30, 2019 and December 31, 2018,
respectively:
|
|
Fair Value
Measurements at September 30, 2019
|
|
|
|
|
|
|
Assets
|
|
|
|
|
Cash
|
$117,209
|
$-
|
$-
|
|
Total
assets
|
117,209
|
-
|
-
|
|
Liabilities
|
|
|
|
|
Convertible notes
payable, net of discounts
|
-
|
192,286
|
-
|
|
Derivative
liabilities
|
-
|
-
|
1,838,652
|
|
Total
liabilities
|
-
|
192,286
|
1,838,652
|
|
|
$117,209
|
$(192,286)
|
$(1,838,652)
|
|
|
Fair Value
Measurements at December 31, 2018
|
|
|
|
|
|
|
Assets
|
|
|
|
|
Cash
|
$86,827
|
$-
|
$-
|
|
Total
assets
|
86,827
|
-
|
-
|
|
Liabilities
|
|
|
|
|
Convertible notes
payable, net of discounts
|
-
|
309,637
|
-
|
|
Derivative
liabilities
|
-
|
-
|
1,690,304
|
|
Total
liabilities
|
-
|
309,637
|
1,690,304
|
|
|
$86,827
|
$(309,637)
|
$(1,690,304)
|
The fair values of
our related party debts are deemed to approximate book value, and
are considered Level 2 inputs as defined by ASC Topic
820-10-35.
There were no
transfers of financial assets or liabilities between Level 1, Level
2 and Level 3 inputs for the nine months ended September 30,
2019 or the year ended December 31, 2018.
Note
5 – Patent Rights and Applications
The Company
amortizes its patent rights and applications on a straight-line
basis over the expected useful technological or economic life of
the patents, which is typically 17 years from the legal approval of
the patent applications when there are probable future economic
benefits associated with the patent. The Company has elected to
expense all of their patent rights and application costs due to
difficulties associated with having to prove the value of their
future economic benefits. All patent applications are currently
pending and the Company has no patents that have yet been approved.
It is the Company’s policy that it performs reviews of the
carrying value of its patent rights and applications on an annual
basis.
On March 4, 2015,
we entered into a Patent License Agreement (“PLA”) with
the University of Texas at El Paso (“UTEP”) regarding
our joint research and development of CTLA-4 Blockade with
Metronomic Chemotherapy for the Treatment of Breast Cancer. This is
the first PLA with UTEP following our Collaborative Agreement with
them dated May 9, 2012, and memorializes the joint
ownership of the applicable patent and the financial and other
terms related thereto.
On June 19, 2015,
we entered into Amendment No. 1 to this Agreement, pursuant to
which we explicitly included Provisional Patent Application No.
62/161,116 entitled, “Anti-CTLA-4 Blockade” (the
“Application”) under the definition of “Patent
Rights” as set forth in the PLA. The Application was filed
with the United States Patent and Trademarks Office on May 13,
2015; the underlying technology was invented by Robert Kirken and
Georgialina Rodriguez, and is solely-owned by The Board of Regents
of The University of Texas System.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
Note
6 – Convertible Notes Payable
Convertible notes
payable consists of the following at September 30, 2019 and
December 31, 2018, respectively:
|
|
|
|
|
|
|
|
|
|
|
|
|
On September 12,
2019, the Company received net proceeds of $22,000, carrying a
$25,750 face value, in exchange for a 12% interest bearing;
unsecured convertible promissory note maturing on
September 12, 2020 (“Third Crown Bridge Partners
Note”). The note is convertible at 60% of the lowest traded
price of the Common Stock in the twenty (20) Trading Days prior to
the Conversion Date. In addition, the holder is entitled to deduct
$500 from the conversion amount in each conversion to cover the
holder’s deposit fees.
|
$25,750
|
$-
|
|
|
|
|
|
On August 15, 2019,
the Company received net proceeds of $40,000, carrying a $43,000
face value, in exchange for a 10% interest bearing; unsecured
convertible promissory note maturing on August 15, 2020
(“Fifth Power Up Lending Note”). The note is
convertible 180 days from the date of the note at 61% of the
average of the two lowest closing bid prices of the Common Stock in
the twenty (20) Trading Days prior to the Conversion
Date.
|
43,000
|
-
|
|
|
|
|
|
On August 2, 2019,
the Company received net proceeds of $35,000, carrying a $38,000
face value, in exchange for a 10% interest bearing; unsecured
convertible promissory note maturing on August 2, 2020
(“Fourth Power Up Lending Note”). The note is
convertible 180 days from the date of the note at 61% of the
average of the two lowest closing bid prices of the Common Stock in
the twenty (20) Trading Days prior to the Conversion
Date.
|
38,000
|
-
|
|
|
|
|
|
On July 2, 2019,
the Company received net proceeds of $31,400, carrying a $36,050
face value, in exchange for a 12% interest bearing; unsecured
convertible promissory note maturing on June 27, 2020
(“Second Crown Bridge Partners Note”). The note is
convertible at 60% of the lowest traded price of the Common Stock
in the twenty (20) Trading Days prior to the Conversion Date. In
addition, the holder is entitled to deduct $500 from the conversion
amount in each conversion to cover the holder’s deposit
fees.
|
36,050
|
-
|
|
|
|
|
|
On June 7, 2019,
the Company received net proceeds of $35,000, carrying a $38,000
face value, in exchange for a 10% interest bearing; unsecured
convertible promissory note maturing on June 7, 2020
(“Third Power Up Lending Note”). The note is
convertible 180 days from the date of the note at 61% of the
average of the two lowest closing bid prices of the Common Stock in
the twenty (20) Trading Days prior to the Conversion
Date.
|
38,000
|
-
|
|
|
|
|
|
On April 23, 2019,
the Company received net proceeds of $35,000, carrying a $38,000
face value, in exchange for a 10% interest bearing; unsecured
convertible promissory note maturing on April 23, 2020
(“Second Power Up Lending Note”). The note is
convertible 180 days from the date of the note at 61% of the
average of the two lowest closing bid prices of the Common Stock in
the twenty (20) Trading Days prior to the Conversion
Date.
|
38,000
|
-
|
|
|
|
|
|
On March 27, 2019,
the Company entered into a securities purchase agreement with Crown
Bridge Partners, LLC to sell convertible notes with a face value of
$154,500, with net proceeds of $141,000 after the deduction of an
original issue discount of $13,500 on a 12% interest bearing;
unsecured convertible promissory note with the first twelve months
of interest of each tranche guaranteed. The maturity date for each
tranche funded shall be twelve (12) months from the effective date
of each payment. The note is payable in tranches with the first
tranche, which was received on April 17, 2019, carrying a $51,500
face value, with net proceeds of $47,000 after a $4,500 original
issue discounts (“First Crown Bridge Partners Note”).
The note is convertible at 60% of the lowest traded price of the
Common Stock in the twenty (20) Trading Days prior to the
Conversion Date. In addition, the holder is entitled to deduct $500
from the conversion amount in each conversion to cover the
holder’s deposit fees.
|
51,500
|
-
|
|
|
|
|
|
On March 26, 2019,
the Company received proceeds of $68,000 in exchange for a 10%
interest bearing; unsecured convertible promissory note maturing on
March 26, 2020 (“First Power Up Lending Note”).
The note is convertible 180 days from the date of the note at 61%
of the average of the two lowest closing bid prices of the Common
Stock in the twenty (20) Trading Days prior to the Conversion Date.
A total of $7,400 of principal was converted into 1,947,368 shares
of common stock on September 30, 2019.
|
60,600
|
-
|
|
|
|
|
|
On July 11, 2018,
the Company received proceeds of $120,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
October 31, 2018 (“Third Red Diamond Note”). The
note is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date. A total of $59,959 of principal was converted into 11,641,667
shares of common stock over various dates between
July 27, 2018 and September 26, 2019. Currently
in default.
|
60,041
|
94,080
|
|
|
|
|
|
On July 11, 2018,
the Company received proceeds of $60,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
October 31, 2018 (“Third SEG-RedaShex Note”). The
note is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date. Currently in default.
|
60,000
|
60,000
|
|
|
|
|
|
On April 24, 2018,
the Company received proceeds of $30,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
July 31, 2018 (“Second Red Diamond Note”). The
note is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date. A total of $32,553, consisting of $30,000 of principal and
$2,553 of interest, was converted into 11,110,400 shares of common
stock over various dates between August 8, 2019 and
September 3, 2019.
|
-
|
30,000
|
|
|
|
|
|
On April 24, 2018,
the Company received proceeds of $30,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
July 31, 2018 (“Second SEG-RedaShex Note”). The
note is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date. A total of $12,636 of principal was converted into 3,510,000
shares of common stock over various dates between
September 10, 2019 and
September 17, 2019.Currently in default.
|
17,364
|
30,000
|
|
|
|
|
|
On March 1, 2018,
the Company received proceeds of $30,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
May 31, 2018 (“First SEG-RedaShex Note”). The note
is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date. A total of $30,000 of principal was converted into an
aggregate of 4,262,416 shares of common stock at various dates
between January 2, 2019 and
August 15, 2019.
|
-
|
30,000
|
|
|
|
|
|
On October 30,
2017, the Company received proceeds of $50,000 in exchange for an
8% interest bearing; unsecured convertible promissory note maturing
on January 31, 2018 (“Second Diamond Rock Note”).
The note is convertible at 60% of the lowest traded price of the
Common Stock in the fifteen (15) Trading Days prior to the
Conversion Date. A $15,000 loss was recognized during the fourth
quarter of 2018 due to the enactment of default provision. A total
of $76,150, consisting of $65,000 of principal and $11,150 of
interest, was converted into 5,169,160 shares of common stock over
various dates between December 12, 2018 and
June 7, 2019.
|
-
|
55,057
|
|
|
|
|
|
On August 8, 2017,
the Company entered into an exchange agreement with Diamond Rock,
LLC whereby they exchanged (i) the 13,333,334 Series A Warrants
purchased in the First Closing, (ii) the 13,333,334 Series B
Warrants purchased in the First Closing, and (iii) the 10,101,011
shares of common stock purchased in the Second Closing (the
“Exchange Securities”) for a $50,000 convertible note
(“First Diamond Rock Note”) issued by the Company,
bearing interest at 8% interest and maturing on November 30, 2017.
The notes are convertible at 50% of the lowest traded price of the
Common Stock in the fifteen (15) Trading Days prior to the
Conversion Date. A $10,500 loss was recognized during the fourth
quarter of 2018 due to the enactment of default provision. A total
of $15,000 of principal was converted into an aggregate of 31,250
shares of common stock at various dates between
November 6, 2017 and November 13, 2017, and
another $35,000 of principal was converted into an aggregate of
751,550 shares of common stock at various dates between
October 12, 2018 and November 30, 2018, along
with $52,581 of principal that was converted into an aggregate of
4,099,700 shares of common stock at various dates between
January 11, 2019 and June 27, 2019. Currently
in default.
|
2,209
|
10,500
|
|
|
|
|
|
Total convertible
notes payable
|
470,514
|
309,637
|
|
Less unamortized
derivative discounts:
|
278,228
|
-
|
|
Convertible notes
payable
|
192,286
|
309,637
|
|
Less: current
portion
|
192,286
|
309,637
|
|
Convertible notes
payable, less current portion
|
$-
|
$-
|
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
In accordance with
ASC 470-20 Debt with Conversion and Other Options, the Company
recorded total discounts of $334,211 and $300,000; including
$29,900 and $-0- of loan origination discounts, for the variable
conversion features of the convertible debts incurred during the
nine months ended September 30, 2019 and the year ended
December 31, 2018, respectively. The discounts are being
amortized to interest expense over the term of the debentures using
the effective interest method. The Company recorded $59,556 and
$306,442 of interest expense pursuant to the amortization of note
discounts during the nine months ended September 30, 2019
and 2018, respectively.
All of the
convertible debentures carry default provisions that place a
“maximum share amount” on the note holders. The maximum
share amount that can be owned as a result of the conversions to
common stock by the note holders is 4.99% of the Company’s
issued and outstanding shares.
In accordance with
ASC 815-15, the Company determined that the variable conversion
feature and shares to be issued on the Redwood Notes represented
embedded derivative features, and these are shown as derivative
liabilities on the balance sheet. The Company calculated the fair
value of the compound embedded derivatives associated with the
convertible debentures utilizing a lattice model.
The Company
recognized interest expense for the nine months ended
September 30, 2019 and 2018, respectively, as
follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest on
convertible notes
|
$40,753
|
$15,881
|
|
Amortization of
debt discounts
|
59,556
|
306,442
|
|
Interest on credit
cards
|
1,484
|
-
|
|
Total interest
expense
|
$101,793
|
$322,323
|
Note
7 – Derivative Liabilities
As
discussed in Note 6 under Convertible Notes Payable, the Company
issued debts that consist of the issuance of convertible notes with
variable conversion provisions. The conversion terms of the
convertible notes are variable based on certain factors, such as
the future price of the Company’s common stock. The number of
shares of common stock to be issued is based on the future price of
the Company’s common stock. The number of shares of common
stock issuable upon conversion of the promissory note is
indeterminate. Due to the fact that the number of shares of common
stock issuable could exceed the Company’s authorized share
limit, the equity environment is tainted and all additional
convertible debentures and warrants are included in the value of
the derivative. Pursuant to ASC 815-15 Embedded Derivatives, the
fair values of the variable conversion option and warrants and
shares to be issued were recorded as derivative liabilities on the
issuance date.
The fair values of the Company’s derivative
liabilities were estimated at the issuance date and are revalued at
each subsequent reporting date, using a lattice model. The Company
recognized current derivative liabilities of $1,838,652 and
$1,690,304 at September 30, 2019 and
December 31, 2018, respectively. The change in fair value
of the derivative liabilities resulted in a loss of $18,690 and a
gain of $655,808 for the nine months ended September 30, 2019
and 2018, respectively, which has been reported within other
income in the statements of operations. The loss of $18,690 for the
nine months ended September 30, 2019 consisted of a gain of
$26,267 due to the value attributable to the warrants and a loss in
market value of $44,957 on the convertible notes. The gain of $655,808 for the nine months ended
September 30, 2018 consisted of a gain of $743,751 due to
the value attributable to the warrants and a net loss in market
value of $87,943 on the convertible notes.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
The following is a
summary of changes in the fair market value of the derivative
liability during the nine months ended
September 30, 2019 and the year ended
December 31, 2018, respectively:
|
|
|
|
|
|
|
|
|
|
Balance, December
31, 2017
|
$2,255,781
|
|
Increase
in derivative value due to issuances of convertible promissory
notes
|
336,643
|
|
Change
in fair market value of derivative liabilities due to the mark to
market adjustment
|
(702,493)
|
|
Debt
conversions
|
(199,627)
|
|
Balance, December
31, 2018
|
$1,690,304
|
|
Increase
in derivative value due to issuances of convertible promissory
notes
|
307,884
|
|
Change
in fair market value of derivative liabilities due to the mark to
market adjustment
|
18,690
|
|
Debt
conversions
|
(178,226)
|
|
Balance,
September 30, 2019
|
$1,838,652
|
Key inputs and assumptions used to value the convertible debentures
and warrants issued during the nine months ended September 30,
2019:
●
Stock price
ranging from $0.0285 to $0.0066 during these periods would
fluctuate with projected volatility.
●
The
notes convert with variable conversion prices and fixed conversion
prices (tainted notes).
●
An
event of default would occur -0-% of the time, increasing 2% per
month to a maximum of 10%.
●
The
projected annual volatility curve for each valuation period was
based on the historical annual volatility of the company in the
range of 246.5% - 452.3%.
●
The
Company would redeem the notes -0-% of the time, increasing 1% per
month to a maximum of 5%.
●
All
notes are assumed to be extended at maturity – the time
required to convert out this volume of stock.
●
A
change of control and fundamental transaction would occur initially
-0-% of the time and increase monthly by -0-% to a maximum of
-0-%.
●
The
monthly trading volume would average $336,476 to $357,240 and would
increase at 1% per month.
●
The stock price
would fluctuate with the Company projected volatility using a
random sampling (500,000 iterations for each valuation) from a
normal distribution. The stock price of the underlying instrument
is modelled such that it follows a geometric Brownian motion with
constant drift and volatility.
●
The Holder would
exercise the warrants after one trading day as they become
exercisable (at issuance) at target prices of 3 to 5 times the
projected reset price or higher.
●
Reset events were
projected to occur by 9/30/19 – the option expires
3/31/20.
●
The stock price
would fluctuate with an annual volatility. The projected annual
volatility curve for each valuation period was based on the
historical annual volatility of the company and the term remaining
in the range 369.2% - 369.2%.
●
The Holder would
exercise the warrant at maturity in 2020 if the stock price was
above the reset exercise price.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
Note
8 – Commitments and Contingencies
Collaborative Patent License Agreements
On May 9, 2012, the
Company entered into a Collaborative Agreement with the University
of Texas at El Paso. Pursuant to the terms of the Agreement, the
Company will work jointly with the University to develop a series
of research and development programs around its sequential-dialysis
technology in the areas of Alzheimer's Disease, Traumatic Brain
Injury (TBI), Chronic Pain Syndrome, Fibromyalgia, Multiple
Sclerosis, Amyotrophic Lateral Sclerosis (ALS or Lou Gehrig's
disease), Blood Sepsis, Cancer, Heart Attacks and Strokes. The
programs will utilize the facilities at one or more of the
University of Texas’ campuses. The Company will pay the
University’s actual overhead for the projects, plus a
negotiated facility and administration overhead expense, and 10% of
all gross revenues associated with the sale, license and/or
royalties of all products and treatment procedures directly
affiliated with programs. Intellectual property jointly invented
and developed as a result of the projects will be owned jointly by
the University and the Company. The Agreement has an initial term
of five (5) years, and is renewable upon mutual agreement of the
parties.
On March 4, 2015,
we entered into a Patent License Agreement (PLA) with the
University of Texas at El Paso (UTEP) regarding our joint research
and development of CTLA-4 Blockade with Metronomic Chemotherapy for
the Treatment of Breast Cancer. This is the first PLA with UTEP
following our Collaborative Agreement with them dated
May 9, 2012, and memorializes the joint ownership of the
applicable patent and the financial and other terms related
thereto.
On June 19, 2015,
we entered into Amendment No. 1 to this Agreement, pursuant to
which we explicitly included Provisional Patent Application No.
62/161,116 entitled, “Anti-CTLA-4 Blockade” (the
“Application”) under the definition of “Patent
Rights” as set forth in the PLA. The Application was filed
with the United States Patent and Trademarks Office on
May 13, 2015; the underlying technology was invented by
Robert Kirken and Georgialina Rodriguez, and is solely-owned by The
Board of Regents of The University of Texas System.
On October 31, 2017
we entered into an Agreement, Final Payment under Contract, and
Release of all Claims, whereby we agreed to pay them a total of
$326,336 arising out of the research and development agreements
with an initial payment of $22,211, and monthly payments of varying
amounts between $5,000 and $20,000 thereafter for twenty eight
months until the balance is paid in full. Subject to the compliance
of all terms, the intellectual property rights established and
arising out of the collaborative agreements remain in full force
and effect and the parties agreed to a mutual release upon the
final contracted payment. The full amount of the liability has been
recognized as accounts payable, with $155,024 outstanding as of the
end of this period, which is currently in default.
Note
9 – Changes in Stockholders’ Equity
(Deficit)
Reverse Stock Split
On June 27, 2018, the Company effected a
1-for-250 reverse stock split (the “Reverse Stock
Split”). No fractional
shares were issued, and no cash or other consideration was paid in
connection with the Reverse Stock Split. Instead, the Company
issued one whole share of the post-Reverse Stock Split common stock
to any stockholder who otherwise would have received a fractional
share as a result of the Reverse Stock Split. The Company was
authorized to issue 1,000,000,000 shares of common stock prior to
the Reverse Stock Split, which remains unaffected. The Reverse
Stock Split did not have any effect on the stated par value of the
common stock, or the Company’s authorized preferred stock.
Unless otherwise stated, all share and per share information in
this Quarterly Report on Form 10-Q has been retroactively adjusted
to reflect the Reverse Stock Split.
Convertible Preferred Stock
The Company has
10,000,000 authorized shares of Preferred Stock, of which 2,000,000
shares of $0.001 par value Series A Convertible Preferred Stock
(“Series A Preferred Stock”) have been designated, and
another 1,000,000 shares of $0.001 par value Series B Convertible
Preferred Stock (“Series B Preferred Stock”) were
designated on November 23, 2018. The Company shall reserve and
keep available out of its authorized but unissued shares of Common
Stock such number of shares sufficient to effect the conversions,
and agreed to reserve no less than 225 million
shares.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
Convertible Preferred Stock, Series A
Each share of
Series A Preferred Stock is convertible, at the option of the
holder thereof, at any time after the issuance of such share into
one (1) fully paid and non-assessable share of Common Stock. Each
outstanding share of Series A Preferred Stock is entitled to one
hundred (100) votes per share on all matters to which the
shareholders of the Corporation are entitled or required to
vote.
Convertible Preferred Stock, Series B
Each share of
Series B Preferred Stock is convertible, at the option of the
holder thereof, at any time after the issuance of such share
into that number of fully paid and
nonassessable shares of our common stock equal to the quotient of
the Conversion Principal Amount divided by the lesser of (a) the
Fixed Conversion Price established by our Board of Directors on the
date of conversion, and (b) the Fair Market Value. The Certificate
of Designation defines Fair Market Value as 60% of the lowest
Traded Price for the common stock for the previous fifteen (15)
trading days prior to the Conversion Date on the market or exchange
where our common stock is trading. The Conversion Principal Amount
is equal to the Original Issue Price ($1.00) divided by nine-tenths
(0.9). The Fixed Conversion Price is the price set by our Board of
Directors upon conversion but in no event less than the last Traded
Price of our common stock. Traded Price is defined as the price at
which our common stock changes hands on the designated exchange or
market. Conversion of the
Series B Preferred Stock is subject to a Beneficial Ownership
Limitation that prohibits the conversion of the Series B Preferred
Stock if the conversion would result in beneficial ownership by the
holder and its affiliates of more than 4.99% of our outstanding
shares of common stock. A holder of Series B Preferred Stock may
increase its Beneficial Ownership Limitation up to 9.99% but only
after 61 days have passed since the holder gave notice to the
Company. The Series B Preferred
Stock has no voting rights. The rights of the Series B Preferred
Stock survive any reorganization, merger or sale of the
Company.
The
holders of Series B Preferred Stock shall receive noncumulative
dividends on an as-converted basis in the same form as any
dividends to be paid out on shares of our common stock. Any
dividends paid will first be paid to the holders of Series B
Preferred Stock prior and in preference to any payment or
distribution to holders of common stock. Other than as set forth in
the previous sentence, the Certificate of Designation provides that
no other dividends shall be paid on Series B Preferred Stock.
Dividends on the Series B Preferred Stock are not mandatory or
cumulative. There are no sinking fund provisions applicable to the
Series B Preferred Stock, and the holders of Series B Preferred
Stock have no redemption rights. The Corporation may redeem the
Series B Preferred Stock upon 30 days’ prior notice at a
price equal to the sum of 133% of the Original Issue Price plus the
amount of any unpaid dividends on the shares to be
redeemed.
As
long as any shares of Series B Preferred Stock remain outstanding,
the Certificate of Designation provides that without the approval
of 75% of the holders of the outstanding Series B Preferred Stock,
we may not (i) alter or change the rights, preferences, or
privileges of the Series B Convertible Preferred Stock, (ii)
increase or decrease the number of authorized shares of Series B
Convertible Preferred Stock, or (iii) authorize the issuance of
securities having a preference over or on par with the Series B
Preferred Stock.
Common Stock Issuances for Series B Preferred Stock
Conversions
On August 8, 2019,
the Company issued 925,927 shares of common stock pursuant to the
conversion of 2,500 of Series B Convertible Preferred Stock
held by RedDiamond Partners.
On August 2, 2019,
the Company issued 851,853 shares of common stock pursuant to the
conversion of 2,300 of Series B Convertible Preferred Stock
held by RedDiamond Partners.
On July 29, 2019,
the Company issued 796,297 shares of common stock pursuant to the
conversion of 2,150 of Series B Convertible Preferred Stock
held by RedDiamond Partners.
On July 23, 2019,
the Company issued 741,741 shares of common stock pursuant to the
conversion of 2,470 of Series B Convertible Preferred Stock
held by RedDiamond Partners.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
On July 16, 2019,
the Company issued 707,071 shares of common stock pursuant to the
conversion of 3,500 of Series B Convertible Preferred Stock
held by RedDiamond Partners.
On July 8, 2019,
the Company issued 666,667 shares of common stock pursuant to the
conversion of 3,300 of Series B Convertible Preferred Stock
held by RedDiamond Partners.
Common Stock
The Company has one
billion authorized shares of $0.00001 par value Common Stock, as
increased pursuant to an amendment to the articles of incorporation
on February 9, 2016.
Common Stock Issuances for Debt Conversions
On September 30,
2019, the Company issued 1,947,368 shares of common stock pursuant
to the conversion of $7,400 of principal from the First PowerUp
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On September 26,
2019, the Company issued 2,150,000 shares of common stock pursuant
to the conversion of $6,450 of principal from the Third RedDiamond
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On September 23,
2019, the Company issued 2,050,000 shares of common stock pursuant
to the conversion of $6,150 of principal from the Third RedDiamond
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On September 18,
2019, the Company issued 1,950,000 shares of common stock pursuant
to the conversion of $5,850 of principal from the Third RedDiamond
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On September 17,
2019, the Company issued 1,920,000 shares of common stock pursuant
to the conversion of $6,912 of principal from the Second
SEG-RedaShex Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On September 16,
2019, the Company issued 1,863,000 shares of common stock pursuant
to the conversion of $5,589 of principal from the Third RedDiamond
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On September 12,
2019, the Company issued 1,680,000 shares of common stock pursuant
to the conversion of $5,040 of principal from the Third RedDiamond
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On September 10,
2019, the Company issued 1,590,000 shares of common stock pursuant
to the conversion of $5,724 of principal from the Second
SEG-RedaShex Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On September 6,
2019, the Company issued 1,600,000 shares of common stock pursuant
to the conversion of $4,960 of principal from the Third RedDiamond
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On September 3,
2019, the Company issued 1,540,000 shares of common stock pursuant
to the conversion of $4,774, consisting of $2,221 of principal and
$2,553 of interest from the Second RedDiamond Note. The note was
converted in accordance with the conversion terms; therefore, no
gain or loss has been recognized.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
On August 28, 2019,
the Company issued 1,469,000 shares of common stock pursuant to the
conversion of $4,554 of principal from the Second RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 22, 2019,
the Company issued 1,360,000 shares of common stock pursuant to the
conversion of $4,216 of principal from the Second RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 20, 2019,
the Company issued 1,295,000 shares of common stock pursuant to the
conversion of $4,533 of principal from the Second RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 19, 2019,
the Company issued 1,230,000 shares of common stock pursuant to the
conversion of $3,936 of principal from the Second RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 15, 2019,
the Company issued 1,124,000 shares of common stock pursuant to the
conversion of $2,810 of principal from the Second RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 15, 2019,
the Company issued 833,333 shares of common stock pursuant to the
conversion of $2,500 of principal from the First SEG-RedaShex Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 14, 2019,
the Company issued 1,080,000 shares of common stock pursuant to the
conversion of $2,700 of principal from the Second RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 13, 2019,
the Company issued 1,030,000 shares of common stock pursuant to the
conversion of $2,575 of principal from the Second RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 12, 2019,
the Company issued 833,333 shares of common stock pursuant to the
conversion of $2,500 of principal from the First SEG-RedaShex Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 12, 2019,
the Company issued 982,400 shares of common stock pursuant to the
conversion of $2,456 of principal from the Second RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On August 1, 2019,
the Company issued 833,333 shares of common stock pursuant to the
conversion of $2,500 of principal from the First SEG-RedaShex Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On July 11, 2019,
the Company issued 634,057 shares of common stock pursuant to the
conversion of $3,500 of principal from the First SEG-RedaShex Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On June 27, 2019,
the Company issued 640,000 shares of common stock pursuant to the
conversion of $2,944 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
On June 21, 2019,
the Company issued 612,500 shares of common stock pursuant to the
conversion of $2,817 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On June 17, 2019,
the Company issued 550,000 shares of common stock pursuant to the
conversion of $2,530 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On June 7, 2019,
the Company issued 530,000 shares of common stock pursuant to the
conversion of $2,703 of interest from the Second Diamond Rock Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On May 28, 2019,
the Company issued 505,000 shares of common stock pursuant to the
conversion of $4,596 of interest from the Second Diamond Rock Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On May 22, 2019,
the Company issued 497,512 shares of common stock pursuant to the
conversion of $6,000 of principal from the First SEG-RedaShex Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On May 16, 2019,
the Company issued 480,000 shares of common stock pursuant to the
conversion of $4,992, consisting of $1,141 of principal and $3,851
of interest, from the Second Diamond Rock Note. The note was
converted in accordance with the conversion terms; therefore, no
gain or loss has been recognized.
On May 7, 2019, the
Company issued 460,000 shares of common stock pursuant to the
conversion of $5,106 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On April 26, 2019,
the Company issued 400,000 shares of common stock pursuant to the
conversion of $4,520 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On March 22, 2019,
the Company issued 386,000 shares of common stock pursuant to the
conversion of $6,369, consisting of $2,136 of principal and $4,233
of interest, from the Second Diamond Rock Note. The note was
converted in accordance with the conversion terms; therefore, no
gain or loss has been recognized.
On March 6, 2019,
the Company issued 370,000 shares of common stock pursuant to the
conversion of $5,739 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On February 26,
2019, the Company issued 349,463 shares of common stock pursuant to
the conversion of $6,500 of principal from the First SEG-RedaShex
Note. The shares were subsequently issued in May of 2019. The note
was converted in accordance with the conversion terms; therefore,
no gain or loss has been recognized.
On February 26,
2019, the Company issued 340,000 shares of common stock pursuant to
the conversion of $5,273 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On February 12,
2019, the Company issued 346,200 shares of common stock pursuant to
the conversion of $6,924 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
On February 1,
2019, the Company issued 315,000 shares of common stock pursuant to
the conversion of $7,875 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On January 23,
2019, the Company issued 260,000 shares of common stock pursuant to
the conversion of $6,513 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On January 11,
2019, the Company issued 280,000 shares of common stock pursuant to
the conversion of $5,597 of principal from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On January 2, 2019,
the Company issued 281,385 shares of common stock pursuant to the
conversion of $6,500 of principal from the First SEG-RedaShex Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
Common Stock Issuances on Subscriptions Payable
On January 1, 2019,
the Company issued 276,960 shares to
DiamondRock, LLC for the conversion of $5,345 of debt on
December 31, 2018.
Note
10 – Income Taxes
The Company
accounts for income taxes under FASB ASC 740-10, which requires use
of the liability method. FASB ASC 740-10-25 provides that deferred
tax assets and liabilities are recorded based on the differences
between the tax bases of assets and liabilities and their carrying
amounts for financial reporting purposes, referred to as temporary
differences.
For the nine months
ended September 30, 2019, and the year ended December 31,
2018, the Company incurred a net operating loss and, accordingly,
no provision for income taxes has been recorded. In addition, no
benefit for income taxes has been recorded due to the uncertainty
of the realization of any tax assets. At September 30, 2019,
and December 31, 2018, the Company had approximately
$5,540,000 and $5,277,000 of federal net operating losses,
respectively. The net operating loss carry forwards, if not
utilized, will begin to expire in 2031.
The components of
the Company’s deferred tax asset are as follows:
|
|
|
|
|
|
|
|
|
Deferred tax
assets:
|
|
|
|
Net operating loss
carry forwards
|
$1,163,400
|
$1,108,170
|
|
|
|
|
|
Net deferred tax
assets before valuation allowance
|
$1,163,400
|
$1,108,170
|
|
Less: Valuation
allowance
|
(1,163,400)
|
(1,108,170)
|
|
Net deferred tax
assets
|
$-
|
$-
|
Based on the
available objective evidence, including the Company’s history
of losses, management believes it is more likely than not that the
net deferred tax assets will not be fully realizable. Accordingly,
the Company provided for a full valuation allowance against its net
deferred tax assets at September 30, 2019, and December 31,
2018, respectively.
Premier
Biomedical, Inc.
Notes to Condensed
Financial Statements
(Unaudited)
A reconciliation
between the amounts of income tax benefit determined by applying
the applicable U.S. and state statutory income tax rate to pre-tax
loss is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal and state
statutory rate
|
21%
|
21%
|
|
Change in valuation
allowance on deferred tax assets
|
(21%)
|
(21%)
|
In accordance with
FASB ASC 740, the Company has evaluated its tax positions and
determined there are no uncertain tax positions.
Note
11 – Subsequent Events
Convertible Debt Financing
On October 3, 2019,
the Company received net proceeds of $25,000, carrying a $150,000
face value after a $125,000 commitment fee, pursuant to the first
tranche of the securities purchase agreement with Green Coast
Capital International SA (“First GCCI Note”) on a 12%
interest bearing; unsecured convertible promissory note; maturing
on October 3, 2020, with the first twelve (12) months of interest
guaranteed. The note is convertible at 60% of the lowest traded
price of the Common Stock in the fifteen (15) Trading Days prior to
the Conversion Date. In addition, the holder is entitled to deduct
$1,000 from the conversion amount in each conversion to cover the
holder’s deposit fees.
Common Stock Issuances for Debt Conversions
On various dates
from October 4, 2019 through November 11, 2019, the Company issued
a total of 48,950,000 shares of common stock pursuant to the
conversion of $46,374 of principal from the Third RedDiamond Note.
The note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On various dates
from October 28, 2019 through November 12, 2019, the Company issued
a total of 45,969,063 shares of common stock pursuant to the
conversion of $39,900, consisting of $38,000 of principal and
$1,900 of interest, from the Second PowerUp Note. The note was
converted in accordance with the conversion terms; therefore, no
gain or loss has been recognized.
On various dates
from October 2, 2019 through October 21, 2019, the Company issued a
total of 27,225,607 shares of common stock pursuant to the
conversion of $64,000 of convertible debt, consisting of $60,600 of
principal and $3,400 of interest, from the First PowerUp Note. The
note was converted in accordance with the conversion terms;
therefore, no gain or loss has been recognized.
On various dates
from October 18, 2019 through November 5, 2019, the Company issued
a total of 15,600,000 shares of common stock pursuant to the
conversion of $8,860 of principal and $1,500 of selling of fees,
from the First Crown Bridge Note. The note was converted in
accordance with the conversion terms; therefore, no gain or loss
has been recognized.

REPORT OF INDEPENDENT REGISTERED PUBLIC
ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Premier Biomedical, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Premier
Biomedical, Inc. (the Company) as of December 31, 2018 and 2017,
and the related statements of operations, stockholders’
equity (deficit), and cash flows for each of the years in the
two-year period ended December 31, 2018, and the related notes and
schedules (collectively referred to as the financial statements).
In our opinion, the financial statements present fairly, in all
material respects, the financial position of the Company as of
December 31, 2018 and 2017, and the results of its operations and
its cash flows for each of the years in the two-year period ended
December 31, 2018, in conformity with accounting principles
generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public
Company Accounting Oversight Board (United States) (PCAOB) and are
required to be independent with respect to the Company in
accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and
the PCAOB.
We conducted our audits in accordance with the standards of the
PCAOB. Those standards require that we plan and perform the audit
to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error
or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial
reporting. As part of our audits, we are required to obtain an
understanding of internal control over financial reporting, but not
for the purpose of expressing an opinion on the effectiveness of
the Company’s internal control over financial reporting.
Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of
material misstatement of the financial statements, whether due to
error or fraud, and performing procedures that respond to those
risks. Such procedures included examining, on a test basis,
evidence regarding the amounts and disclosures in the financial
statements. Our audits also included evaluating the accounting
principles used and significant estimates made by management, as
well as evaluating the overall presentation of the financial
statements. We believe that our audits provide a reasonable basis
for our opinion.
The accompanying financial statements have been prepared assuming
the Company will continue as a going concern. As discussed in Note
2 to the financial statements, the Company suffered losses from
operations which raise substantial doubt about its ability to
continue as a going concern. Managements plans regarding those
matters are also described in Note 2. The financial statements do
not include any adjustments that might result from the outcome of
this uncertainty.
/s/ M&K CPAS, PLLC
We have served as the Company’s auditor since
2011.
Houston, TX
April 15, 2019
BALANCE SHEETS
|
|
|
|
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
Current
assets:
|
|
|
|
Cash
|
$86,827
|
$83,704
|
|
Accounts
receivable
|
3,092
|
312
|
|
Inventory
|
25,985
|
84,763
|
|
Other current
assets
|
43,883
|
34,824
|
|
Total current
assets
|
159,787
|
203,603
|
|
|
|
|
|
Property and
equipment, net
|
5,203
|
5,478
|
|
|
|
|
|
Total
assets
|
$164,990
|
$209,081
|
|
|
|
|
|
|
|
|
|
LIABILITIES
AND STOCKHOLDERS' EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
Accounts
payable
|
$264,398
|
$346,814
|
|
Accounts payable,
related parties
|
25,944
|
41,382
|
|
Accrued
interest
|
22,099
|
5,840
|
|
Convertible notes
payable, net of discounts of $-0- and $30,010
|
|
|
|
at December 31,
2018 and 2017, respectively, currently in default
|
309,637
|
169,990
|
|
Derivative
liabilities
|
1,690,304
|
2,255,781
|
|
Total current
liabilities
|
2,312,382
|
2,819,807
|
|
|
|
|
|
Total
liabilities
|
2,312,382
|
2,819,807
|
|
|
|
|
|
Commitments and
contingencies
|
-
|
-
|
|
|
|
|
|
Stockholders'
equity (deficit):
|
|
|
|
Series A
convertible preferred stock, $0.001 par value, 10,000,000 shares
authorized,
|
|
|
|
2,000,000 shares
designated, issued and outstanding at December 31, 2018 and
2017
|
2,000
|
2,000
|
Series
B convertible preferred stock, $0.001 par value, 1,000,000 shares
designated, 150,000
|
|
|
and -0- shares
issued and outstanding at December 31, 2018 and 2017,
respectively
|
150
|
-
|
|
Common stock,
$0.00001 par value, 1,000,000,000 shares
|
|
|
|
authorized,
5,652,410 and 2,551,363 shares issued and
|
|
|
|
outstanding at
December 31, 2018 and 2017, respectively
|
57
|
26
|
|
Additional paid in
capital
|
14,572,754
|
13,442,255
|
|
Subscriptions
payable, consisting of 276,960 and 254,703
|
|
|
|
shares at December
31, 2018 and 2017, respectively
|
5,345
|
273,805
|
|
Accumulated
deficit
|
(16,727,698)
|
(16,328,812)
|
|
Total stockholders'
equity (deficit)
|
(2,147,392)
|
(2,610,726)
|
|
|
|
|
|
Total liabilities
and stockholders' equity (deficit)
|
$164,990
|
$209,081
|
The
accompanying notes are an integral part of these financial
statements.
STATEMENTS OF OPERATIONS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue
|
$39,795
|
$39,761
|
|
Cost of goods
sold
|
113,727
|
25,439
|
|
Gross profit
(loss)
|
(73,932)
|
14,322
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
Research and
development
|
-
|
184,315
|
|
General and
administrative
|
189,285
|
196,670
|
|
Professional
fees
|
429,625
|
923,175
|
|
Total operating
expenses
|
618,910
|
1,304,160
|
|
|
|
|
|
Net operating
loss
|
(692,842)
|
(1,289,838)
|
|
|
|
|
|
Other income
(expense):
|
|
|
|
Interest
expense
|
(415,287)
|
(351,502)
|
|
Gain on early
extinguishment of debt
|
6,750
|
-
|
|
Change in
derivative liabilities
|
702,493
|
(2,115,986)
|
|
Loss on joint
venture
|
-
|
(6,232)
|
|
Total other income
(expense)
|
293,956
|
(2,473,720)
|
|
|
|
|
|
Net
loss
|
$(398,886)
|
$(3,763,558)
|
|
|
|
|
|
|
|
|
|
Weighted average
number of common shares
|
|
|
|
outstanding - basic
and fully diluted
|
3,505,460
|
1,958,745
|
|
|
|
|
|
Net loss per share
- basic and fully diluted
|
$(0.11)
|
$(1.92)
|
The
accompanying notes are an integral part of these financial
statements.
STATEMENT OF STOCKHOLDERS' EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31,
2016
|
2,000,000
|
$2,000
|
-
|
$-
|
1,218,227
|
$13
|
$11,902,537
|
$-
|
$(12,565,254)
|
$(660,704)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock sold for
cash
|
-
|
-
|
-
|
-
|
160,000
|
2
|
284,998
|
-
|
-
|
285,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued on equity line
of credit
|
-
|
-
|
-
|
-
|
20,588
|
-
|
18,323
|
-
|
-
|
18,323
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued on debt
conversions
|
-
|
-
|
-
|
-
|
797,368
|
8
|
423,189
|
-
|
-
|
423,197
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of warrants at $0.00001
per share, related parties
|
-
|
-
|
-
|
-
|
28,000
|
-
|
70
|
-
|
-
|
70
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued for services on
terminated offering
|
-
|
-
|
-
|
-
|
291,180
|
3
|
313,015
|
273,805
|
-
|
586,823
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued for
services
|
-
|
-
|
-
|
-
|
36,000
|
-
|
84,600
|
-
|
-
|
84,600
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for services,
related parties
|
-
|
-
|
-
|
-
|
-
|
-
|
102,364
|
-
|
-
|
102,364
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for
services
|
-
|
-
|
-
|
-
|
-
|
-
|
9,617
|
-
|
-
|
9,617
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to derivative liability
due to debt conversions
|
-
|
-
|
-
|
-
|
-
|
-
|
303,542
|
-
|
-
|
303,542
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the year ended
December 31, 2017
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
(3,763,558)
|
(3,763,558)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31,
2017
|
2,000,000
|
$2,000
|
-
|
$-
|
2,551,363
|
$26
|
$13,442,255
|
$273,805
|
$(16,328,812)
|
$(2,610,726)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued on
subsctiptions payable
|
-
|
-
|
-
|
-
|
254,703
|
3
|
273,802
|
(273,805)
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series B convertible preferred
stock sold for cash
|
-
|
-
|
150,000
|
150
|
-
|
-
|
149,850
|
-
|
-
|
150,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued on debt
conversions
|
-
|
-
|
-
|
-
|
2,834,264
|
28
|
210,246
|
5,345
|
-
|
215,619
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of warrants at $0.00001
per share, related parties
|
-
|
-
|
-
|
-
|
12,000
|
-
|
30
|
-
|
-
|
30
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Odd lot shares issued on reverse
stock split
|
-
|
-
|
-
|
-
|
80
|
-
|
-
|
-
|
-
|
-
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for services,
related parties
|
-
|
-
|
-
|
-
|
-
|
-
|
272,585
|
-
|
-
|
272,585
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for
services
|
-
|
-
|
-
|
-
|
-
|
-
|
24,359
|
-
|
-
|
24,359
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to derivative liability
due to debt conversions
|
-
|
-
|
-
|
-
|
-
|
-
|
199,627
|
-
|
-
|
199,627
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss for the year ended
December 31, 2018
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
(398,886)
|
(398,886)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31,
2018
|
2,000,000
|
$2,000
|
150,000
|
$150
|
5,652,410
|
$57
|
$14,572,754
|
$5,345
|
$(16,727,698)
|
$(2,147,392)
|
The
accompanying notes are an integral part of these financial
statements.
STATEMENTS OF CASH FLOWS
|
|
|
|
|
|
|
|
|
|
|
CASH
FLOWS FROM OPERATING ACTIVITIES
|
|
|
|
Net
loss
|
$(398,886)
|
$(3,763,558)
|
|
Adjustments to
reconcile net loss
|
|
|
|
to net cash used in
operating activities:
|
|
|
|
Allowance for
inventory obsolescence
|
87,650
|
2,316
|
|
Depreciation
|
2,304
|
2,316
|
|
Gain on early
extinguishment of debt
|
(6,750)
|
-
|
|
Loss on debt
default provisions
|
25,500
|
-
|
|
Change in fair
market value of derivative liabilities
|
(702,493)
|
2,115,986
|
|
Amortization of
debt discounts
|
366,653
|
340,961
|
|
Stock based
compensation, related parties
|
272,585
|
102,364
|
|
Stock based
compensation
|
24,359
|
681,040
|
|
Decrease (increase)
in assets:
|
|
|
|
Accounts
receivable
|
(2,780)
|
(312)
|
|
Inventory
|
(28,872)
|
(84,763)
|
|
Other current
assets
|
(9,059)
|
(23,394)
|
|
Increase (decrease)
in liabilities:
|
|
|
|
Accounts
payable
|
(82,416)
|
126,074
|
|
Accounts payable,
related parties
|
(15,438)
|
(11,107)
|
|
Accrued
interest
|
22,765
|
8,531
|
|
Accrued interest,
related parties
|
-
|
(3,570)
|
|
Net cash used in
operating activities
|
(444,878)
|
(507,116)
|
|
|
|
|
|
CASH
FLOWS FROM INVESTING ACTIVITIES
|
|
|
|
Purchases of
property and equipment
|
(2,029)
|
(2,694)
|
|
Net cash used in
investing activities
|
(2,029)
|
(2,694)
|
|
|
|
|
|
CASH
FLOWS FROM FINANCING ACTIVITIES
|
|
|
|
Proceeds from sale
of stock, net of offering costs
|
150,000
|
285,000
|
|
Proceeds from sale
of stock on equity line of credit
|
-
|
18,323
|
|
Proceeds from
exercise of warrants, related party
|
30
|
70
|
|
Proceeds from
convertible notes payable
|
300,000
|
300,000
|
|
Repayments of notes
payable, related parties
|
-
|
(30,000)
|
|
Net cash provided
by financing activities
|
450,030
|
573,393
|
|
|
|
|
|
NET CHANGE IN
CASH
|
3,123
|
63,583
|
|
CASH AT BEGINNING
OF PERIOD
|
83,704
|
22,437
|
|
|
|
|
|
CASH AT END OF
PERIOD
|
$86,827
|
$86,020
|
|
|
|
|
|
SUPPLEMENTAL
INFORMATION:
|
|
|
|
Interest
paid
|
$369
|
$5,580
|
|
Income taxes
paid
|
$-
|
$-
|
|
|
|
|
|
NON-CASH INVESTING
AND FINANCING ACTIVITIES:
|
|
|
|
Value of debt
discounts
|
$300,000
|
$221,515
|
|
Value of derivative
adjustment due to debt conversions
|
$199,627
|
$303,542
|
|
Value of shares
issued for conversion of debt
|
$215,619
|
$423,197
|
The
accompanying notes are an integral part of these financial
statements.
Notes to Financial
Statements
Note 1 – Nature of Business and Significant Accounting
Policies
Nature of
Business
Premier
Biomedical, Inc. (“the Company”) was incorporated in
the State of Nevada on May 10, 2010 (“Inception”). The
Company was formed to develop and market medications and procedures
that address a significant number of the most highly visible health
issues currently affecting mankind. Our current focus is primarily
on the development and distribution of our pain
products.
These
statements reflect all adjustments, consisting of normal recurring
adjustments, which in the opinion of management are necessary for
fair presentation of the information contained
therein.
Use of
Estimates
The
preparation of financial statements in conformity with accounting
principles generally accepted in the United States requires
management to make estimates and assumptions that affect the
reported amounts of assets and liabilities, and the disclosure of
contingent assets and liabilities at the date of the financial
statements, and the reported amounts of revenues and expenses
during the reporting period. Actual results could differ from those
estimates.
Cash and Cash
Equivalents
We
maintain cash balances in non-interest-bearing accounts, which do
not currently exceed federally insured limits. For the purpose of
the statements of cash flows, all highly liquid investments with an
original maturity of three months or less are considered to be cash
equivalents.
Patent Rights and
Applications
Patent
rights and applications costs include the acquisition costs and
costs incurred for the filing of patents. Patent rights and
applications are amortized on a straight-line basis over the legal
life of the patent rights beginning at the time the patents are
approved. Patent costs for unsuccessful patent applications are
expensed when the application is terminated.
Fair Value of Financial
Instruments
Under
FASB ASC 820-10-05, the Financial Accounting Standards Board
establishes a framework for measuring fair value in generally
accepted accounting principles and expands disclosures about fair
value measurements. This Statement reaffirms that fair value is the
relevant measurement attribute. The adoption of this standard did
not have a material effect on the Company’s financial
statements as reflected herein. The carrying amounts of cash,
prepaid expenses and accrued expenses reported on the balance sheet
are estimated by management to approximate fair value primarily due
to the short term nature of the instruments.
Basic and Diluted Loss Per
Share
The
basic net loss per common share is computed by dividing the net
loss by the weighted average number of common shares outstanding.
Diluted net loss per common share is computed by dividing the net
loss adjusted on an “as if converted” basis, by the
weighted average number of common shares outstanding plus potential
dilutive securities. For the periods presented, potential dilutive
securities had an anti-dilutive effect and were not included in the
calculation of diluted net loss per common share.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Stock-Based
Compensation
Under
FASB ASC 718-10-30-2, all share-based payments to employees,
including grants of employee stock options, to be recognized in the
income statement based on their fair values. Pro forma disclosure
is no longer an alternative. The Company’s stock-based
compensation consisted of the following during the years ended
December 31, 2018 and 2017,
respectively:
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued
for services
|
$-
|
$84,600
|
|
Warrants issued for
services, related parties
|
272,585
|
102,364
|
|
Warrants issued for
services
|
24,359
|
9,617
|
|
Common stock issued
for services on terminated offering
|
-
|
586,823
|
|
Total stock based
compensation
|
$296,944
|
$783,404
|
Revenue
Recognition
On January 1, 2018, we adopted Accounting Standards Update No.
2014-09, Revenue from Contracts with Customers (Topic 606), which
supersedes the revenue recognition requirements in Accounting
Standards Codification (ASC) Topic 605, Revenue Recognition (Topic
605). Results for reporting periods beginning after January 1, 2018
are presented under Topic 606. The impact of adopting the new
revenue standard was not material to our financial statements and
there was no adjustment to beginning retained earnings on January
1, 2018.
Under Topic 606, revenue is recognized when control of the promised
goods or services is transferred to our customers, in an amount
that reflects the consideration we expect to be entitled to in
exchange for those goods or services.
We determine revenue recognition through the following
steps:
●
identification
of the contract, or contracts, with a customer;
●
identification
of the performance obligations in the contract;
●
determination
of the transaction price;
●
allocation
of the transaction price to the performance obligations in the
contract; and
●
recognition
of revenue when, or as, we satisfy a performance
obligation.
Sales are recorded when the earnings process is complete or
substantially complete, and the revenue is measurable and
collectability is reasonably assured, which is typically when
products are shipped. Provisions for discounts and rebates to
customers, estimated returns and allowances, and other adjustments
are provided for in the same period the related sales are recorded.
The Company defers any revenue from sales in which payment has been
received, but the earnings process has not been completed. Sales
commenced on July 5, 2017 with the termination of our joint
venture.
Advertising and
Promotion
All
costs associated with advertising and promoting products are
expensed as incurred. These expenses were $66,244 and $64,108 for
the years ended December 31, 2018 and 2017,
respectively.
Income Taxes
Deferred
tax assets and liabilities are recognized for the future tax
consequences attributable to differences between the financial
statement carrying amounts of existing assets and liabilities and
their respective tax bases. Deferred tax assets and liabilities are
measured using enacted tax rates expected to apply to taxable
income in the years in which those temporary differences are
expected to be recovered or settled. A valuation allowance is
provided for significant deferred tax assets when it is more likely
than not, that such asset will not be recovered through future
operations.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Uncertain Tax
Positions
In accordance with ASC 740, “Income Taxes” (“ASC
740”), the Company recognizes the tax benefit from an
uncertain tax position only if it is more likely than not that the
tax position will be capable of withstanding examination by the
taxing authorities based on the technical merits of the position.
These standards prescribe a recognition threshold and measurement
attribute for the financial statement recognition and measurement
of a tax position taken or expected to be taken in a tax return.
These standards also provide guidance on de-recognition,
classification, interest and penalties, accounting in interim
periods, disclosure, and transition.
Various taxing authorities periodically audit the Company’s
income tax returns. These audits include questions regarding the
Company’s tax filing positions, including the timing and
amount of deductions and the allocation of income to various tax
jurisdictions. In evaluating the exposures connected with these
various tax filing positions, including state and local taxes, the
Company records allowances for probable exposures. A number of
years may elapse before a particular matter, for which an allowance
has been established, is audited and fully resolved. The Company
has not yet undergone an examination by any taxing
authorities.
The assessment of the Company’s tax position relies on the
judgment of management to estimate the exposures associated with
the Company’s various filing positions.
Recently Issued Accounting
Pronouncements
In
August 2018, the Financial Accounting Standards Board
(“FASB”) issued Accounting Standards Update
(“ASU”) 2018-13, Fair
Measurement (Topic 820): Disclosure Framework – Changes to
the Disclosure Requirements for Fair Value Measurement,
which modify the disclosure requirements of Topic 820. The new
guidance is effective for all entities for annual periods, and
interim periods within those annual periods, beginning after
December 15, 2019, with early adoption permitted. The Company does
not expect the adoption of this ASU to have a material impact on
its consolidated financial statements.
In June
2018, the FASB issued ASU 2018-07, Compensation-Stock Compensation (Topic 718):
Improvements to Nonemployee Share-Based Payment Accounting,
which expands the scope of Topic 718 to include share-based payment
transactions for acquiring goods and services from nonemployees. An
entity should apply the requirements of Topic 718 to nonemployee
awards except for specific guidance on inputs to an option pricing
model and the attribution of cost (that is, the period of time over
which share-based payment awards vest and the pattern of cost
recognition over that period). The new guidance is effective for
all entities for annual periods, and interim periods within those
annual periods, beginning after December 15, 2017, with early
adoption permitted. The Company does not expect the adoption of
this ASU to have a material impact on its consolidated financial
statements.
In
March 2018, the FASB issued ASU No. 2018-05, Income Taxes (Topic 740) - Amendments to SEC
Paragraphs Pursuant to SEC Staff Accounting Bulletin No.
118. The amendment provides guidance on accounting for the
impact of the Tax Cuts and Jobs Act (the “Tax Act”) and
allows entities to complete the accounting under ASC 740 within a
one-year measurement period from the Tax Act enactment date. This
standard is effective upon issuance. The Tax Act has several
significant changes that impact all taxpayers, including a
transition tax, which is a one-time tax charge on accumulated,
undistributed foreign earnings. The calculation of accumulated
foreign earnings requires an analysis of each foreign
entity’s financial results going back to 1986. The Company
does not expect the adoption of this ASU to have a material impact
on its consolidated financial statements.
In February 2018, the FASB issued ASU No. 2018-02,
Reclassification
of Certain Tax Effects from Accumulated Other Comprehensive
Income. The guidance permits
entities to reclassify tax effects stranded in Accumulated Other
Comprehensive Income as a result of tax reform to retained
earnings. This new guidance is effective for annual and interim
periods in fiscal years beginning after December 15, 2018. Early
adoption is permitted in annual and interim periods and can be
applied retrospectively or in the period of adoption. The Company
is currently in the process of evaluating the impact of adoption on
its consolidated financial statements.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Effective
January 1, 2018, the Company adopted ASC 606 — Revenue from
Contracts with Customers. Under ASC 606, the Company recognizes
revenue from the commercial sales of products, licensing agreements
and contracts to perform pilot studies by applying the following
steps: (1) identify the contract with a customer; (2) identify the
performance obligations in the contract; (3) determine the
transaction price; (4) allocate the transaction price to each
performance obligation in the contract; and (5) recognize revenue
when each performance obligation is satisfied. For the comparative
periods, revenue has not been adjusted and continues to be reported
under ASC 605 — Revenue Recognition. Under ASC 605, revenue
is recognized when the following criteria are met: (1) persuasive
evidence of an arrangement exists; (2) the performance of service
has been rendered to a customer or delivery has occurred; (3) the
amount of fee to be paid by a customer is fixed and determinable;
and (4) the collectability of the fee is reasonably assured.
There was no impact on the
Company’s financial statements as a result of adopting Topic
606 for the years ended December 31, 2018 and
2017.
No
other new accounting pronouncements, issued or effective during the
year ended December 31, 2018, have had or are expected to have a
significant impact on the Company’s financial
statements.
Note 2 – Going Concern
As
shown in the accompanying financial statements, the Company has no
revenues, incurred net losses from operations resulting in an
accumulated deficit of $16,727,698, and had negative working
capital of ($2,152,595) at December 31, 2018. These
factors raise substantial doubt about the Company’s ability
to continue as a going concern. Management is actively pursuing new
products and services to begin generating revenues. In addition,
the Company is currently seeking additional sources of capital to
fund short term operations. The Company, however, is dependent upon
its ability to secure equity and/or debt financing and there are no
assurances that the Company will be successful; therefore, without
sufficient financing it would be unlikely for the Company to
continue as a going concern.
The
financial statements do not include any adjustments that might
result from the outcome of any uncertainty as to the
Company’s ability to continue as a going concern. The
financial statements also do not include any adjustments relating
to the recoverability and classification of recorded asset amounts,
or amounts and classifications of liabilities that might be
necessary should the Company be unable to continue as a going
concern.
Note 3 – Related Party
Accounts Payable
The
Company owed $24,116 and $39,116 as of December 31, 2018 and 2017,
respectively, to entities owned by the Chairman of the Board of
Directors. The amounts are related to patent costs paid by the
Chairman on behalf of the Company.
The
Company owed $753 and $713 as of December 31, 2018 and 2017,
respectively, to the Company’s CEO for reimbursable
expenses.
The
Company owed $1,075 and $1,553 as of December 31, 2018 and 2017,
respectively, amongst members of the Company’s Board of
Directors for reimbursable expenses.
Notes Payable
On July
6, 2015, the Company received an unsecured loan in the amount of
$10,000, due on demand, bearing interest at a simple interest rate
of 8%, from the Company’s CEO. The principal and interest was
repaid in full in November of 2017.
On July
6, 2015, the Company received an unsecured loan in the amount of
$10,000, due on demand, bearing interest at a simple interest rate
of 8%, from the Company’s Chairman of the Board. The
principal and interest was repaid in full in November of
2017.
On July
6, 2015, the Company received an unsecured loan in the amount of
$10,000, due on demand, bearing interest at a simple interest rate
of 8%, from one of the Company’s Directors. The principal and
interest was repaid in full in November of 2017.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Common Stock Warrants Exercised
On
November 5, 2018, the Company issued 12,000 shares of common stock
pursuant to the exercise of warrants by the Company’s
Chairman of the Board at $0.0025 per share for total proceeds of
$30.
On
November 22, 2017, the Company issued 28,000 shares of common stock
pursuant to the exercise of warrants by the Company’s CEO at
$0.0025 per share for total proceeds of $70.
Common Stock Warrants Granted
On
December 15, 2018, the Company granted warrants to the following
officers and directors, which will allow them to purchase shares of
our common stock in the amounts indicated: William Hartman (842,000
shares); Mitchell Felder (842,000 shares), Heidi Carl (500,000
shares), John Borza (579,000 shares), Jay Rosen (52,500 shares),
Patricio Reyes (500,000 shares) and John Pauly (52,500 shares). The
exercise price of the foregoing warrants is nine cents ($0.09) per
share. The warrants are exercisable over seven (7) years. The total
fair value of the 3,368,000 common stock warrants using the
Black-Scholes option-pricing model is $272,585, or $0.08093 per
share, based on a volatility rate of 211%, a risk-free interest
rate of 2.72% and an expected term of 3.5 years, and was expensed
upon issuance.
On
December 22, 2017, the Company granted warrants to the following
officers and directors, which will allow them to purchase shares of
our common stock in the amounts indicated: William Hartman (34,000
shares); Mitchell Felder (34,000 shares), Heidi Carl (24,000
shares), John Borza (29,000 shares), Jay Rosen (4,000 shares),
Patricio Reyes (16,000 shares) and John Pauly (8,000 shares). The
exercise price of the foregoing warrants is one dollar and
twenty-five cents ($1.25) per share. The warrants are exercisable
over seven (7) years. The total fair value of the 149,000 common
stock warrants using the Black-Scholes option-pricing model is
$102,364, or $0.68699 per share, based on a volatility rate of
195%, a risk-free interest rate of 2.01% and an expected term of
3.5 years, and was expensed upon issuance.
Loss on Joint Venture
The
Company advanced a total of $48,778 to its joint venture partner,
Premier Biomedical Pain Management Solutions, LLC, and was
subsequently repaid a total of $44,604 on July 5, 2017 with the
termination of the joint venture, resulting in a loss of $6,232,
consisting of the original investment of $2,058 and the loss on
this receivable of $4,174, for the year ended December 31,
2017.
Note 4 – Joint Venture
On
September 13, 2016, we entered into an operating agreement to form
a pain management joint venture company with Advanced Technologies
Solutions (ATS), a company based in San Diego, California and owned
by Ronald T. LaBorde, a member of our Board of Directors. The joint
venture company, Premier Biomedical Pain Management Solutions, LLC,
a Nevada limited liability company (PBPMS), to develop and market
natural and cannabis-based generalized, neuropathic, and localized
pain relief treatment products. We owned 50% of PBPMS and ATS owned
the other 50%, with 89% of the profits allocated to us and the
remaining 11% of profits allocated to ATS. As part of the agreement
with ATS, Mr. LaBorde was appointed a member of our Board of
Directors.
PBPMS
was required to enter into separate license agreements with us and
ATS for the use of technology previously developed by both
companies. Intellectual property developed jointly by the parties
will be the property of PBPMS. However, ATS and Mr. LaBorde could
have developed inventions and intellectual property independently
from PBPMS, and such inventions and intellectual property would
have been the sole property of ATS or Mr. LaBorde. Pursuant to the
terms of the PBPMS operating agreement, The Company was to tender
1,250,000 warrants, for the purchase of an equal number of shares
of our common stock at a strike price of $0.05, pursuant to the
license agreement between ATS and PBPMS. The Company and Mr.
LaBorde did not execute the license agreement or issue these
warrants, and on July 5, 2017, the Company terminated the joint
venture agreement, resulting in a loss of $6,232.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Our
initial capital contribution to PBPMS was $25,000. ATS was to
contribute (i) technical, labor, manufacturing information and
know-how required to produce the initial product, an extended
duration topical pain relief patch; (ii) $5,000 worth of primary
ingredients; and (iii) $5,000 worth of other materials to produce
the initial prototype pain relief patches.
PBPMS
was managed by a board of managers (PBPMS Board). The PBPMS Board
consisted of William A. Hartman, our President and Chief Executive
Officer and member of our Board of Directors, Ronald T. LaBorde,
the Founder of ATS and member of our Board of Directors, Dr.
Patricio Reyes, our Chief Technology Officer and member of our
Board of Directors, and John Borza, our Vice-President and member
of our Board of Directors. Decisions of the PBPMS Board require
unanimous approval.
The
PBPMS operating agreement was subject to other common terms and
ownership transfer restrictions, including a right of first
refusal; however, the operating agreement and entity were dissolved
upon the termination of the joint venture on July 5,
2017.
Note 5 – Subsidiary Formation
On
September 14, 2017, we formed Premier Biomedical Pain Relief Meds,
LLC as a wholly-owned Nevada limited liability company. On January
1, 2018, we contributed our pain management assets to this entity
and continued our pain management operations within this new
subsidiary.
Note 6 – Fair Value of Financial Instruments
Under
FASB ASC 820-10-5, fair value is defined as the price that would be
received to sell an asset or paid to transfer a liability in an
orderly transaction between market participants at the measurement
date (an exit price). The standard outlines a valuation framework
and creates a fair value hierarchy in order to increase the
consistency and comparability of fair value measurements and the
related disclosures. Under GAAP, certain assets and liabilities
must be measured at fair value, and FASB ASC 820-10-50 details the
disclosures that are required for items measured at fair
value.
The
Company has certain financial instruments that must be measured
under the new fair value standard. The Company’s financial
assets and liabilities are measured using inputs from the three
levels of the fair value hierarchy. The three levels are as
follows:
Level 1
- Inputs are unadjusted quoted prices in active markets for
identical assets or liabilities that the Company has the ability to
access at the measurement date.
Level 2
- Inputs include quoted prices for similar assets and liabilities
in active markets, quoted prices for identical or similar assets or
liabilities in markets that are not active, inputs other than
quoted prices that are observable for the asset or liability (e.g.,
interest rates, yield curves, etc.), and inputs that are derived
principally from or corroborated by observable market data by
correlation or other means (market corroborated
inputs).
Level 3
- Unobservable inputs that reflect our assumptions about the
assumptions that market participants would use in pricing the asset
or liability.
The
following schedule summarizes the valuation of financial
instruments at fair value on a recurring basis in the balance
sheets as of December 31, 2018 and 2017, respectively:
|
|
Fair Value
Measurements at December 31, 2018
|
|
|
|
|
|
|
Assets
|
|
|
|
|
Cash
|
$86,827
|
$-
|
$-
|
|
Total
assets
|
86,827
|
-
|
-
|
|
Liabilities
|
|
|
|
|
Convertible note
payable
|
-
|
309,637
|
-
|
|
Derivative
liabilities
|
-
|
-
|
1,690,304
|
|
Total
liabilities
|
-
|
309,637
|
1,690,304
|
|
|
$86,827
|
$(309,637)
|
$(1,690,304)
|
Premier
Biomedical, Inc.
Notes to Financial
Statements
|
|
Fair Value
Measurements at December 31, 2017
|
|
|
|
|
|
|
Assets
|
|
|
|
|
Cash
|
$83,704
|
$-
|
$-
|
|
Total
assets
|
83,704
|
-
|
-
|
|
Liabilities
|
|
|
|
|
Convertible note
payable, net of discounts
|
-
|
169,990
|
-
|
|
Derivative
liabilities
|
-
|
-
|
2,255,781
|
|
Total
liabilities
|
-
|
169,990
|
2,255,781
|
|
|
$83,704
|
$(169,990)
|
$(2,255,781)
|
The
fair values of our related party debts are deemed to approximate
book value, and are considered Level 2 inputs as defined by
ASC Topic 820-10-35.
There
were no transfers of financial assets or liabilities between Level
1, Level 2 and Level 3 inputs for the years ended December 31, 2018
or the year ended December 31, 2017.
Note 7 – Patent Rights and Applications
The
Company amortizes its patent rights and applications on a
straight-line basis over the expected useful technological or
economic life of the patents, which is typically 17 years from the
legal approval of the patent applications when there are probable
future economic benefits associated with the patent. The Company
has elected to expense all of their patent rights and application
costs due to difficulties associated with having to prove the value
of their future economic benefits. All patent applications are
currently pending and the Company has no patents that have yet been
approved. It is the Company’s policy that it performs reviews
of the carrying value of its patent rights and applications on an
annual basis.
On
March 4, 2015, we entered into a Patent License Agreement
(“PLA”) with the University of Texas at El Paso
(“UTEP”) regarding our joint research and development
of CTLA-4 Blockade with Metronomic Chemotherapy for the Treatment
of Breast Cancer. This is the first PLA with UTEP following our
Collaborative Agreement with them dated May 9, 2012, and
memorializes the joint ownership of the applicable patent and the
financial and other terms related thereto.
On June
19, 2015, we entered into Amendment No. 1 to this Agreement,
pursuant to which we explicitly included Provisional Patent
Application No. 62/161,116 entitled, “Anti-CTLA-4
Blockade” (the “Application”) under the
definition of “Patent Rights” as set forth in the PLA.
The Application was filed with the United States Patent and
Trademarks Office on May 13, 2015; the underlying technology was
invented by Robert Kirken and Georgialina Rodriguez, and is
solely-owned by The Board of Regents of The University of Texas
System.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Note 8 – Convertible Notes Payable
Convertible
notes payable consists of the following at
December 31, 2018 and 2017,
respectively:
|
|
|
|
|
|
|
|
|
|
|
|
|
On July 11, 2018,
the Company received proceeds of $120,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
October 31, 2018 (“Third Red Diamond Note”). The
note is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date. A total of $25,920 of principal was converted into 348,667
shares of common stock over various dates between
July 27, 2018 and August 23, 2018.
|
$94,080
|
$-
|
|
|
|
|
|
On July 11, 2018,
the Company received proceeds of $60,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
October 31, 2018 (“Third SEG-RedaShex Note”). The
note is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date.
|
60,000
|
-
|
|
|
|
|
|
On April 24, 2018,
the Company received proceeds of $30,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
July 31, 2018 (“Second Red Diamond Note”). The
note is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date.
|
30,000
|
-
|
|
|
|
|
|
On April 24, 2018,
the Company received proceeds of $30,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
July 31, 2018 (“Second SEG-RedaShex Note”). The
note is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date.
|
30,000
|
-
|
|
|
|
|
|
On March 1, 2018,
the Company received proceeds of $30,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
May 31, 2018 (“First SEG-RedaShex Note”). The note
is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date.
|
30,000
|
-
|
|
|
|
|
|
On March 1, 2018,
the Company received proceeds of $30,000 in exchange for an 8%
interest bearing; unsecured convertible promissory note maturing on
May 31, 2018 (“First Red Diamond Note”). The note
is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date. A total of $30,000 of principal was converted into 387,815
shares of common stock over various dates between
September 5, 2018 and
October 3, 2018.
|
-
|
-
|
|
|
|
|
|
On October 30,
2017, the Company received proceeds of $50,000 in exchange for an
8% interest bearing; unsecured convertible promissory note maturing
on January 31, 2018 (“Second Diamond Rock Note”).
The note is convertible at 60% of the lowest traded price of the
Common Stock in the fifteen (15) Trading Days prior to the
Conversion Date. A $15,000 loss was recognized during the fourth
quarter of 2018 due to the enactment of default provision. A total
of $9,943 of principal was converted into 496,960 shares of common
stock over various dates between December 12, 2018 and
December 31, 2018, and 276,960 of those shares were
subsequently issued on January 1, 2019.
|
55,057
|
50,000
|
|
|
|
|
|
On October 30,
2017, the Company received proceeds of $50,000 in exchange for an
8% interest bearing; unsecured convertible promissory note maturing
on January 31, 2018 (“Second SEG Note”). The note
is convertible at 60% of the lowest traded price of the Common
Stock in the fifteen (15) Trading Days prior to the Conversion
Date. A total of $10,000 of principal was converted into 20,833
shares of common stock on October 31, 2017, and the
remaining $40,000 of principal was converted into 106,238 shares of
common stock on January 29, 2018.
|
-
|
40,000
|
|
|
|
|
|
On August 8, 2017,
the Company entered into an exchange agreement with Diamond Rock,
LLC whereby they exchanged (i) the 13,333,334 Series A Warrants
purchased in the First Closing, (ii) the 13,333,334 Series B
Warrants purchased in the First Closing, and (iii) the 10,101,011
shares of common stock purchased in the Second Closing (the
“Exchange Securities”) for a $50,000 convertible note
(“First Diamond Rock Note”) issued by the Company,
bearing interest at 8% interest and maturing on November 30, 2017.
The notes are convertible at 50% of the lowest traded price of the
Common Stock in the fifteen (15) Trading Days prior to the
Conversion Date. A $10,500 loss was recognized during the fourth
quarter of 2018 due to the enactment of default provision. A total
of $15,000 of principal was converted into an aggregate of 31,250
shares of common stock at various dates between
November 6, 2017 and November 13, 2017, and
another $35,000 of principal was converted into an aggregate of
751,550 shares of common stock at various dates between
October 12, 2018 and
November 30, 2018.
|
10,500
|
35,000
|
|
|
|
|
|
On August 8, 2017,
the Company entered into an exchange agreement with The Special
Equities Group, LLC whereby they exchanged (i) the 13,333,334
Series A Warrants purchased in the First Closing, (ii) the
13,333,334 Series B Warrants purchased in the First Closing, and
(iii) the 10,101,011 shares of common stock purchased in the Second
Closing (the “Exchange Securities”) for a $50,000
convertible note (“First SEG Note”) issued by the
Company, bearing interest at 8% interest and maturing on November
30, 2017. The notes are convertible at 50% of the lowest traded
price of the Common Stock in the fifteen (15) Trading Days prior to
the Conversion Date. A total of $49,756, consisting of $43,250 of
principal and $6,506 of interest, was converted into 943,071 shares
of common stock over various dates between
August 20, 2018 and December 12, 2018. An
additional $6,750 of principal was forgiven on the
note.
|
-
|
50,000
|
|
|
|
|
|
On August 8, 2017,
the Company entered into an exchange agreement with RDW Capital,
LLC whereby they exchanged (i) the 13,333,334 Series A Warrants
purchased in the First Closing, (ii) the 13,333,334 Series B
Warrants purchased in the First Closing, and (iii) the 10,101,011
shares of common stock purchased in the Second Closing (the
“Exchange Securities”) for a $50,000 convertible note
(“First RDW Note”) issued by the Company, bearing
interest at 8% interest and maturing on November 30, 2017. The
notes are convertible at 50% of the lowest traded price of the
Common Stock in the fifteen (15) Trading Days prior to the
Conversion Date. A total of $25,000 of principal was converted into
52,632 shares of common stock on October 31, 2017, and
the remaining $25,000 of principal was converted into 76,923 shares
of common stock on January 3, 2018.
|
-
|
25,000
|
|
|
|
|
|
Total convertible
notes payable, currently in default
|
309,637
|
200,000
|
|
Less unamortized
derivative discounts:
|
-
|
30,010
|
|
Convertible notes
payable
|
309,637
|
169,990
|
|
Less: current
portion
|
309,637
|
169,990
|
|
Convertible notes
payable, less current portion
|
$-
|
$-
|
Premier
Biomedical, Inc.
Notes to Financial
Statements
In
accordance with ASC 470-20 Debt with Conversion and Other Options,
the Company recorded total discounts of $300,000 and $221,515 for
the variable conversion features of the convertible debts incurred
during the years ended December 31, 2018 and 2017,
respectively. The discounts are being amortized to interest expense
over the term of the debentures using the effective interest
method. The Company recorded $366,653 and $340,961 of interest
expense pursuant to the amortization of note discounts during the
years ended December 31, 2018 and 2017,
respectively.
All of
the convertible debentures carry default provisions that place a
“maximum share amount” on the note holders. The maximum
share amount that can be owned as a result of the conversions to
common stock by the note holders is 4.99% of the Company’s
issued and outstanding shares.
In
accordance with ASC 815-15, the Company determined that the
variable conversion feature and shares to be issued on the Redwood
Notes represented embedded derivative features, and these are shown
as derivative liabilities on the balance sheet. The Company
calculated the fair value of the compound embedded derivatives
associated with the convertible debentures utilizing a lattice
model.
The
Company recognized interest expense for the years ended
December 31, 2018 and 2017, respectively, as
follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest on
convertible notes
|
$22,765
|
$8,531
|
|
Interest on related
party loans
|
-
|
2,010
|
|
Amortization of
derivative discounts
|
366,653
|
340,961
|
|
Loss on default
provisions
|
25,500
|
-
|
|
Interest on credit
cards
|
369
|
-
|
|
Total interest
expense
|
$415,287
|
$351,502
|
Note 9 – Derivative Liabilities
The Company issued debts that consist of the issuance of
convertible notes with variable conversion provisions. The
conversion terms of the convertible notes are variable based on
certain factors, such as the future price of the Company’s
common stock. The number of shares of common stock to be issued is
based on the future price of the Company’s common stock. The
number of shares of common stock issuable upon conversion of the
promissory note is indeterminate. Due to the fact that the number
of shares of common stock issuable could exceed the Company’s
authorized share limit, the equity environment is tainted and all
additional convertible debentures and warrants are included in the
value of the derivative. Pursuant to ASC 815-15 Embedded
Derivatives, the fair values of the variable conversion option and
warrants and shares to be issued were recorded as derivative
liabilities on the issuance date.
The fair values of the Company’s derivative liabilities were
estimated at the issuance date and are revalued at each subsequent
reporting date, using a lattice model. The Company recognized
current derivative liabilities of $1,690,304 and $2,255,781
at December 31, 2018 and 2017, respectively. The
change in fair value of the derivative liabilities resulted in a
gain of $702,493 and a loss of $2,115,986 for the years ended
December 31, 2018 and 2017, respectively, which has
been reported within other expense in the statements of operations.
The gain of $702,493 for the year ended December 31, 2018
consisted of a net loss in market value of $64,139 on the
convertible notes and a net gain of $766,632 in market value of the
warrants. The loss of $2,115,986 for the year ended
December 31, 2017 consisted of a loss of $7,103,444
attributable to the fair value of warrants, a gain of $3,766,437
due to the subsequent exchange of the warrants, a net loss in
market value of $4,767 on the convertible notes and a net gain of
$1,225,788 in market value of the warrants.
Premier
Biomedical, Inc.
Notes to Financial
Statements
The
following is a summary of changes in the fair market value of the
derivative liability during the years
ended December 31, 2018 and 2017,
respectively:
|
|
|
|
|
|
|
|
|
|
Balance, December
31, 2016
|
$221,822
|
|
Increase
in derivative value due to issuances of convertible promissory
notes
|
221,515
|
|
Increase
in derivative value attributable to tainted warrants
|
7,103,444
|
|
Decrease
in derivative value attributable to exchange of
warrants
|
(3,766,437)
|
|
Change
in fair market value of derivative liabilities due to the mark to
market adjustment
|
(1,221,021)
|
|
Debt
conversions
|
(303,542)
|
|
Balance, December
31, 2017
|
$2,255,781
|
|
Increase
in derivative value due to issuances of convertible promissory
notes
|
336,643
|
|
Change
in fair market value of derivative liabilities due to the mark to
market adjustment
|
(702,493)
|
|
Debt
conversions
|
(199,627)
|
|
Balance,
December 31, 2018
|
$1,690,304
|
Key inputs and assumptions used to value the convertible debentures
and warrants issued during the years ended
December 31, 2018 and 2017:
●
Stock price
ranging from $0.19 to $0.0812 during these periods would fluctuate
with projected volatility.
●
The
notes convert with variable conversion prices and fixed conversion
prices (tainted notes).
●
An
event of default would occur -0-% of the time, increasing 2% per
month to a maximum of 10%.
●
The
projected annual volatility curve for each valuation period was
based on the historical annual volatility of the company in the
range of 208.5% - 289.6%.
●
The
Company would redeem the notes -0-% of the time, increasing 1% per
month to a maximum of 5%.
●
All
notes are assumed to be extended at maturity by 2 years – the
time required to convert out this volume of stock.
●
The
holders of the securities would automatically convert midway
through to maturity on a monthly basis based on ownership and
trading volume limitations.
●
A
change of control and fundamental transaction would occur initially
-0-% of the time and increase monthly by -0-% to a maximum of
-0-%.
●
The
monthly trading volume would average $868,383 to $798,331 and would
increase at 1% per month.
●
The stock price
would fluctuate with the Company projected volatility using a
random sampling (500,000 iterations for each valuation) from a
normal distribution. The stock price of the underlying instrument
is modelled such that it follows a geometric Brownian motion with
constant drift and volatility.
●
The Holder would
exercise the warrants after one trading day as they become
exercisable (at issuance) at target prices of 3 to 5 times the
projected reset price or higher.
●
Reset events were
projected to occur by 12/31/18 – the reset provision ends
3/30/19 and the option expires 3/30/20.
●
The stock price
would fluctuate with an annual volatility. The projected annual
volatility curve for each valuation period was based on the
historical annual volatility of the company and the term remaining
in the range 226.2% - 226.2%.
●
The Holder would
exercise the warrant at maturity in 2020 if the stock price was
above the reset exercise price.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Note 10 – Commitments and Contingencies
Collaborative Patent License Agreements
On May
9, 2012, the Company entered into a Collaborative Agreement with
the University of Texas at El Paso. Pursuant to the terms of the
Agreement, the Company will work jointly with the University to
develop a series of research and development programs around its
sequential-dialysis technology in the areas of Alzheimer's Disease,
Traumatic Brain Injury (TBI), Chronic Pain Syndrome, Fibromyalgia,
Multiple Sclerosis, Amyotrophic Lateral Sclerosis (ALS or Lou
Gehrig's disease), Blood Sepsis, Cancer, Heart Attacks and Strokes.
The programs will utilize the facilities at one or more of the
University of Texas’ campuses. The Company will pay the
University’s actual overhead for the projects, plus a
negotiated facility and administration overhead expense, and 10% of
all gross revenues associated with the sale, license and/or
royalties of all products and treatment procedures directly
affiliated with programs. Intellectual property jointly invented
and developed as a result of the projects will be owned jointly by
the University and the Company. The Agreement has an initial term
of five (5) years, and is renewable upon mutual agreement of the
parties.
On
March 4, 2015, we entered into a Patent License Agreement (PLA)
with the University of Texas at El Paso (UTEP) regarding our joint
research and development of CTLA-4 Blockade with Metronomic
Chemotherapy for the Treatment of Breast Cancer. This is the first
PLA with UTEP following our Collaborative Agreement with them dated
May 9, 2012, and memorializes the joint ownership of the
applicable patent and the financial and other terms related
thereto.
On June
19, 2015, we entered into Amendment No. 1 to this Agreement,
pursuant to which we explicitly included Provisional Patent
Application No. 62/161,116 entitled, “Anti-CTLA-4
Blockade” (the “Application”) under the
definition of “Patent Rights” as set forth in the PLA.
The Application was filed with the United States Patent and
Trademarks Office on May 13, 2015; the underlying
technology was invented by Robert Kirken and Georgialina Rodriguez,
and is solely-owned by The Board of Regents of The University of
Texas System.
On
October 31, 2017 we entered into an Agreement, Final Payment under
Contract, and Release of all Claims, whereby we agreed to pay them
a total of $326,336 arising out of the research and development
agreements with an initial payment of $22,211, and monthly payments
of varying amounts between $5,000 and $20,000 thereafter for twenty
eight months until the balance is paid in full. Subject to the
compliance of all terms, the intellectual property rights
established and arising out of the collaborative agreements remain
in full force and effect and the parties agreed to a mutual release
upon the final contracted payment. The full amount of the liability
has been recognized as accounts payable, with $225,024 outstanding
as of the date of this filing, which is currently in
default.
Note 11 – Stockholders’ Equity
Reverse Stock Split
On June 27, 2018, the Company effected a 1-for-250 reverse
stock split (the “Reverse Stock Split”). No fractional shares were issued, and no
cash or other consideration was paid in connection with the Reverse
Stock Split. Instead, the Company issued one whole share of the
post-Reverse Stock Split common stock to any stockholder who
otherwise would have received a fractional share as a result of the
Reverse Stock Split. The Company was authorized to issue
1,000,000,000 shares of common stock prior to the Reverse Stock
Split, which remains unaffected. The Reverse Stock Split did not
have any effect on the stated par value of the common stock, or the
Company’s authorized preferred stock. Unless otherwise
stated, all share and per share information in this Annual Report
on Form 10-K has been retroactively adjusted to reflect the Reverse
Stock Split.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Convertible Preferred Stock
The
Company has 10,000,000 authorized shares of Preferred Stock, of
which 2,000,000 shares of $0.001 par value Series A Convertible
Preferred Stock (“Series A Preferred Stock”) have been
designated, and another 1,000,000 shares of $0.001 par value Series
B Convertible Preferred Stock (“Series B Preferred
Stock”) were designated on November 23, 2018. The Company
shall reserve and keep available out of its authorized but unissued
shares of Common Stock such number of shares sufficient to effect
the conversions, and agreed to reserve no less than 225 million
shares.
Convertible Preferred Stock, Series A
Each
share of Series A Preferred Stock is convertible, at the option of
the holder thereof, at any time after the issuance of such share
into one (1) fully paid and non-assessable share of Common Stock.
Each outstanding share of Series A Preferred Stock is entitled to
one hundred (100) votes per share on all matters to which the
shareholders of the Corporation are entitled or required to
vote.
Convertible Preferred Stock, Series B
Each
share of Series B Preferred Stock is convertible, at the option of
the holder thereof, at any time after the issuance of such share
into that number of fully paid and
nonassessable shares of our common stock equal to the quotient of
the Conversion Principal Amount divided by the lesser of (a) the
Fixed Conversion Price established by our Board of Directors on the
date of conversion, and (b) the Fair Market Value. The Certificate
of Designation defines Fair Market Value as 60% of the lowest
Traded Price for the common stock for the previous fifteen (15)
trading days prior to the Conversion Date on the market or exchange
where our common stock is trading. The Conversion Principal Amount
is equal to the Original Issue Price ($1.00) divided by nine-tenths
(0.9). The Fixed Conversion Price is the price set by our Board of
Directors upon conversion but in no event less than the last Traded
Price of our common stock. Traded Price is defined as the price at
which our common stock changes hands on the designated exchange or
market. Conversion of the
Series B Preferred Stock is subject to a Beneficial Ownership
Limitation that prohibits the conversion of the Series B Preferred
Stock if the conversion would result in beneficial ownership by the
holder and its affiliates of more than 4.99% of our outstanding
shares of common stock. A holder of Series B Preferred Stock may
increase its Beneficial Ownership Limitation up to 9.99% but only
after 61 days have passed since the holder gave notice to the
Company. The Series B Preferred
Stock has no voting rights. The rights of the Series B Preferred
Stock survive any reorganization, merger or sale of the
Company.
The holders of Series B Preferred Stock shall receive noncumulative
dividends on an as-converted basis in the same form as any
dividends to be paid out on shares of our common stock. Any
dividends paid will first be paid to the holders of Series B
Preferred Stock prior and in preference to any payment or
distribution to holders of common stock. Other than as set forth in
the previous sentence, the Certificate of Designation provides that
no other dividends shall be paid on Series B Preferred Stock.
Dividends on the Series B Preferred Stock are not mandatory or
cumulative. There are no sinking fund provisions applicable to the
Series B Preferred Stock, and the holders of Series B Preferred
Stock have no redemption rights. The Corporation may redeem the
Series B Preferred Stock upon 30 days’ prior notice at a
price equal to the sum of 133% of the Original Issue Price plus the
amount of any unpaid dividends on the shares to be
redeemed.
As long as any shares of Series B Preferred Stock remain
outstanding, the Certificate of Designation provides that without
the approval of 75% of the holders of the outstanding Series B
Preferred Stock, we may not (i) alter or change the rights,
preferences, or privileges of the Series B Convertible Preferred
Stock, (ii) increase or decrease the number of authorized shares of
Series B Convertible Preferred Stock, or (iii) authorize the
issuance of securities having a preference over or on par with the
Series B Preferred Stock.
Sale of Convertible Preferred Stock, Series B (2018)
On November 23, 2018, we sold 75,000 shares of Series B
Convertible Preferred Stock to RedDiamond Partners LLC, and another 75,000 shares
of Series B Convertible Preferred Stock to SEG-RedaShex, LLC for $150,000 in total. Pursuant
to the sale, the purchasers have the right to participate in any
future financing up to 100% of the financing for the next 12
months. We also agreed to refrain from issuing any shares of common
stock or equivalents for 30 days after the sale. The agreement also
prohibits the Company from entering into any agreement involving a
Variable Rate Transaction for eight months after the sale. In
addition, we agreed to grant the purchasers a most-favored nation
provision whereby the purchasers may exchange their shares of
Series B Preferred Stock for securities issued in a subsequent
financing on the same terms and conditions. The purchasers also
have anti-dilution rights that allow them to acquire shares of
common stock at a lower conversion price if a person acquires
shares of our common stock or equivalents at a price per share
lower than the conversion price of the Series B Preferred
Stock.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Common Stock
The
Company has one billion authorized shares of $0.00001 par value
Common Stock, as increased pursuant to an amendment to the articles
of incorporation on February 9, 2016.
Common Stock Warrants Exercised (2018)
On
November 5, 2018, the Company issued 12,000 shares of common stock
pursuant to the exercise of warrants by the Company’s
Chairman of the Board at $0.0025 per share for total proceeds of
$30.
Common Stock Warrants Exercised (2017)
On
November 22, 2017, the Company issued 28,000 shares of common stock
pursuant to the exercise of warrants by the Company’s CEO at
$0.0025 per share for total proceeds of $70.
Securities Purchase Agreement (2017)
On
March 30, 2017, we entered into a Securities Purchase Agreement
(the “Purchase Agreement”) by and between the Company
and each of The Special Equities Group, LLC, RDW Capital LLC, and
DiamondRock, LLC (each a “Purchaser” and collectively,
the “Purchasers”) to sell our common stock and warrants
at a fixed price. Pursuant to the Purchase Agreement, we received
from the Purchasers an aggregate of $300,000, net of $15,000 of
offering costs, in exchange for 160,000 shares of our common stock,
warrants to purchase up to 160,000 shares of our common stock at an
exercise price of $7.50 (“Series A Warrants”) and
warrants to purchase up to 160,000 shares or our common stock at an
exercise price of $12.50 (“Series B Warrants”). Both
the Series A Warrants and Series B Warrants issued pursuant to the
Purchase Agreement are exercisable immediately upon receipt and
have a term of three years. In addition, the Purchaser is entitled
to a one-time price reset on the purchase price of the common stock
of each tranche to the lower of (i) $5.00 or (ii) a 50% discount to
the average of the three lowest closing prices in the 20 trading
days prior to the reset date, which is the earlier of (i) the 7
month anniversary of the closing of each tranche of this
transaction or (ii) 20 trading days after the effectiveness of each
tranche. The embedded value in this reset provision is disclosed
further in Note 8.
On May
30, 2017, the Purchasers bought additional shares of our common
stock and warrants for $150,000 (the “Second Closing”),
in exchange for 30,303,033 shares of our common stock, warrants to
purchase up to 121,212 shares of our common stock at an exercise
price of $7.50 (“Series A Warrants”) and warrants to
purchase up to 121,212 shares or our common stock at an exercise
price of $12.50 (“Series B Warrants”). Both the Series
A Warrants and Series B Warrants issued pursuant to the Purchase
Agreement are exercisable immediately upon receipt and have a term
of three years.
The per
share purchase price of the Second Closing was the lesser of (i)
$5.00, subject to certain adjustments for stock splits and other
similar transactions, or (ii) 50% of the closing price on the
trading day immediately prior to the date of sale. The total number
of shares to be sold in the Second Closing were determined by
dividing the total purchase amount of each closing (i.e., $150,000)
by the per share purchase price.
The
Purchase Agreement limits each Purchaser to beneficial ownership of
our common stock of no more than 9.99%. The Purchasers also have
certain anti-dilution rights in the Purchase Agreement for a period
of 12 months. These rights allow the Purchasers to exchange their
shares of common stock received pursuant to the Purchase Agreement
for additional shares on the same terms and conditions of a
subsequent financing.
On
August 8, 2017, the Company and each of the three Purchasers also
entered into exchange agreements whereby the Purchasers exchanged
(i) the 53,333 Series A Warrants purchased in the First Closing,
(ii) the 53,333 Series B Warrants purchased in the First Closing,
and (iii) the 40,404 shares of common stock purchased in the Second
Closing (the “Exchange Securities”) for a $50,000
convertible note (aggregate $150,000) issued by the Company,
bearing interest at 8% interest and maturing on November 30, 2017.
The notes are convertible at 50% of the lowest traded price of the
Common Stock in the fifteen (15) Trading Days prior to the
Conversion Date.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Registration Rights Agreement (2017)
On
March 30, 2017, we entered into a Registration Rights Agreement
with the Purchasers in connection with the Purchase Agreement. In
the Registration Rights Agreement, we agreed to prepare and file a
registration statement with the Securities and Exchange Commission
covering the resale of all of the shares of common stock sold to
the Purchasers and the shares issuable upon exercise of the Series
A Warrants and Series B Warrants. We agreed to file an initial
registration statement as promptly as possible and have it declared
effective no later than June 28, 2017 (or July 28, 2017 if the
registration statement was reviewed by the Securities and Exchange
Commission) and keep it continuously effective until the securities
are sold or may be sold under Rule 144 of the Securities Act
without volume or manner-of-sale restrictions. If all of the
securities cannot be registered on one registration statement, we
agreed to file subsequent registration statements to register the
remaining securities as promptly as allowed. The registration
statement was subsequently withdrawn on July 24, 2017 and the
Purchase Agreement was amended on August 8, 2017 to change the
terms of the third closing to an aggregate of $150,000 of
convertible notes, bearing interest at 8%, convertible at 50% of
the lowest traded price of the Common Stock in the fifteen (15)
Trading Days prior to the Conversion Date.
Common Stock Issuances for Debt Conversions (2018)
On
December 31, 2018, the Company granted 276,960 shares of common
stock pursuant to the conversion of $5,345 of principal from the
Second Diamond Rock Note. The shares were subsequently issued on
January 1, 2019. As such, the $5,345 was presented as a
subscription payable at December 31, 2018. The note was
converted in accordance with the conversion terms; therefore, no
gain or loss has been recognized.
On
December 12, 2018, the Company issued 258,193 shares of common
stock pursuant to the conversion of $6,506 of interest from the
First SEG Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
December 12, 2018, the Company issued 220,000 shares of common
stock pursuant to the conversion of $4,598 of principal from the
Second Diamond Rock Note. The note was converted in accordance with
the conversion terms; therefore, no gain or loss has been
recognized.
On
November 30, 2018, the Company issued 211,550 shares of common
stock pursuant to the conversion of $8,462 of principal from the
First Diamond Rock Note. The note was converted in accordance with
the conversion terms; therefore, no gain or loss has been
recognized.
On
November 12, 2018, the Company issued 150,000 shares of common
stock pursuant to the conversion of $7,650 of principal from the
First SEG Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
November 5, 2018, the Company issued 190,000 shares of common stock
pursuant to the conversion of $8,075 of principal from the First
Diamond Rock Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
October 25, 2018, the Company issued 175,000 shares of common stock
pursuant to the conversion of $8,750 of principal from the First
Diamond Rock Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
October 16, 2018, the Company issued 202,702 shares of common stock
pursuant to the conversion of $13,500 of principal from the First
SEG Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On
October 12, 2018, the Company issued 175,000 shares of common stock
pursuant to the conversion of $9,712 of principal from the First
Diamond Rock Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
October 3, 2018, the Company issued 111,940 shares of common stock
pursuant to the conversion of $7,500 of principal from the First
Diamond Rock Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
Premier
Biomedical, Inc.
Notes to Financial
Statements
On
September 24, 2018, the Company issued 172,176 shares of common
stock pursuant to the conversion of $12,500 of principal from the
First SEG Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
September 18, 2018, the Company issued 136,986 shares of common
stock pursuant to the conversion of $10,000 of principal from the
First Red Diamond Note. The note was converted in accordance with
the conversion terms; therefore, no gain or loss has been
recognized.
On
September 5, 2018, the Company issued 138,889 shares of common
stock pursuant to the conversion of $12,500 of principal from the
First Red Diamond Note. The note was converted in accordance with
the conversion terms; therefore, no gain or loss has been
recognized.
On
August 23, 2018, the Company issued 82,001 shares of common stock
pursuant to the conversion of $4,920 of principal from the Third
Red Diamond Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
August 20, 2018, the Company issued 160,000 shares of common stock
pursuant to the conversion of $9,600 of principal from the First
SEG Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On
August 15, 2018, the Company issued 100,000 shares of common stock
pursuant to the conversion of $6,000 of principal from the Third
Red Diamond Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
August 9, 2018, the Company issued 83,333 shares of common stock
pursuant to the conversion of $5,000 of principal from the Third
Red Diamond Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On July
27, 2018, the Company issued 83,333 shares of common stock pursuant
to the conversion of $10,000 of principal from the Third Red
Diamond Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
January 29, 2018, the Company issued 106,238 shares of common stock
pursuant to the conversion of $40,000 of principal from the Second
SEG Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On
January 3, 2018, the Company issued 76,923 shares of common stock
pursuant to the conversion of $25,000 of principal from the First
RDW Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
Common Stock Issuances for Debt Conversions (2017)
On
various dates between October 31, 2017 and December 26, 2017, the
Company issued a total of 228,775 shares of common stock pursuant
to the conversion of an aggregate of $100,000 of principal, among
the First and Second RDW, SEG and Diamond Rock Notes. The notes
were converted in accordance with the conversion terms; therefore
no gain or loss has been recognized.
On
various dates between January 10, 2017 and March 13, 2017, the
Company issued a total of 568,593 shares of common stock pursuant
to the conversion of an aggregate of $323,197, consisting of
$302,480 of principal and $20,717 of interest, among the Second,
Fifth and Seventh Redwood Notes. The notes were converted in
accordance with the conversion terms; therefore no gain or loss has
been recognized.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Common Stock Issuances on Stock Purchase Agreement
(2017)
On
February 13, 2017, the Company drew down $8,000 on their Stock
Purchase Agreement entered into on May 27, 2016, with Redwood and
issued 8,000 shares of common stock pursuant to the Seventh Put
Notice.
On
January 10, 2017, the Company drew down $10,323 on their Stock
Purchase Agreement entered into on May 27, 2016, with Redwood and
issued 12,588 shares of common stock pursuant to the Sixth Put
Notice.
Common Stock Issuances for Services (2017)
On December 6, 2017, we agreed to issue a total of 545,882 shares
of our common stock to The Special Equities Group, LLC, RDW
Capital, LLC and DiamondRock, LLC as compensation, of which a total
of 291,180 shares were issued immediately with an aggregate fair
value of $313,018 based on the closing price of the
Company’s common stock on the date of grant, and the other
254,703 shares were subsequently issued on various dates between
January 17, 2018 and February 13, 2018. The aggregate fair value of the shares issued
subsequent to December 31, 2017 was $273,805.
On August 25, 2017, we agreed to issue 36,000 shares of our common
stock to a third-party for consulting services rendered. The shares
were subsequently issued on December 1, 2017. The total fair
value of the common stock was $84,600 based on the closing price of
the Company’s common stock on the date of grant.
Common Stock Issuances on Subscriptions Payable (2018)
On
various dates from January 17, 2018 through February 13, 2018, the
Company issued a total of 254,703 shares to The Special Equities Group and DiamondRock, LLC
as compensation valued at $273,805 awarded on
December 6, 2017.
Note 12 – Common Stock Warrants
Common Stock Warrants Granted (2018)
On
December 15, 2018, the Company granted warrants to the following
officers and directors, which will allow them to purchase shares of
our common stock in the amounts indicated: William Hartman (842,000
shares); Mitchell Felder (842,000 shares), Heidi Carl (500,000
shares), John Borza (579,000 shares), Jay Rosen (52,500 shares),
Patricio Reyes (500,000 shares) and John Pauly (52,500 shares). The
exercise price of the foregoing warrants is nine cents ($0.09) per
share. The warrants are exercisable over seven (7) years. The total
fair value of the 3,368,000 common stock warrants using the
Black-Scholes option-pricing model is $272,585, or $0.08093 per
share, based on a volatility rate of 211%, a risk-free interest
rate of 2.72% and an expected term of 3.5 years, and was expensed
upon issuance.
On
December 15, 2018, we also issued warrants to purchase a total of
two hundred and eighty-eight thousand (288,000) shares of our
common stock amongst four members of our Scientific Advisory Board.
The exercise price of the foregoing warrants is nine cents ($0.09)
per share. The warrants are exercisable over seven (7) years. The
total fair value of the 288,000 common stock warrants using the
Black-Scholes option-pricing model is $24,359, or $0.08458 per
share, based on a volatility rate of 211%, a risk-free interest
rate of 2.81% and an expected term of 7 years, and was expensed
upon issuance.
Common Stock Warrants Granted (2017)
On
December 22, 2017, the Company granted warrants to the following
officers and directors, which will allow them to purchase shares of
our common stock in the amounts indicated: William Hartman (34,000
shares); Mitchell Felder (34,000 shares), Heidi Carl (24,000
shares), John Borza (29,000 shares), Jay Rosen (4,000 shares),
Patricio Reyes (16,000 shares) and John Pauly (8,000 shares). The
exercise price of the foregoing warrants is one dollar and
twenty-five cents ($1.25) per share. The warrants are exercisable
over seven (7) years. The total fair value of the 149,000 common
stock warrants using the Black-Scholes option-pricing model is
$102,364, or $0.68699 per share, based on a volatility rate of
195%, a risk-free interest rate of 2.01% and an expected term of
3.5 years, and was expensed upon issuance.
Premier
Biomedical, Inc.
Notes to Financial
Statements
On
December 22, 2017, we also issued warrants to purchase a total of
fourteen thousand (14,000) shares of our common stock amongst three
members of our Scientific Advisory Board. The exercise price of the
foregoing warrants is one dollar and twenty-five cents ($1.25) per
share. The warrants are exercisable over seven (7) years. The total
fair value of the 14,000 common stock warrants using the
Black-Scholes option-pricing model is $9,617, or $0.68699 per
share, based on a volatility rate of 195%, a risk-free interest
rate of 2.01% and an expected term of 7 years, and was expensed
upon issuance.
A total of $296,944 and $111,981 of warrants were amortized and
expensed to professional fees as stock-based compensation during
the years ended December 31, 2018 and 2017,
respectively, including $272,585 and $102,364 during the years
ended December 31, 2018 and 2017, respectively,
related to warrants issued to related parties.
Exercise of Common Stock Warrants, Related Party
(2018)
On
November 5, 2018, the Company issued 12,000 shares of common stock
pursuant to the exercise of warrants by the Company’s
Chairman of the Board at $0.0025 per share for total proceeds of
$30.
Exercise of Common Stock Warrants, Related Party
(2017)
On
November 22, 2017, the Company issued 28,000 shares of common stock
pursuant to the exercise of warrants by the Company’s CEO at
$0.0025 per share for total proceeds of $70.
The
following is a summary of information about the Common Stock
Warrants outstanding at December 31, 2018.
|
|
|
|
Shares
Underlying
|
|
|
Shares
Underlying Warrants Outstanding
|
|
Warrants
Exercisable
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
Shares
|
|
Average
|
|
Weighted
|
|
Shares
|
|
Weighted
|
|
Range
of
|
|
Underlying
|
|
Remaining
|
|
Average
|
|
Underlying
|
|
Average
|
|
Exercise
|
|
Warrants
|
|
Contractual
|
|
Exercise
|
|
Warrants
|
|
Exercise
|
|
Prices
|
|
Outstanding
|
|
Life
|
|
Price
|
|
Exercisable
|
|
Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$1.25
– $362.50
|
|
3,901,760
|
|
6.85
years
|
|
$2.05
|
|
3,901,760
|
|
$2.05
|
The
fair value of each warrant grant is estimated on the date of grant
using the Black-Scholes option pricing model with the following
weighted-average assumptions used for grants under the fixed option
plan:
|
|
|
|
|
|
|
|
|
|
|
|
|
Average risk-free
interest rates
|
2.73%
|
1.75%
|
|
Average expected
life (in years)
|
3.78
|
9.22
|
The
Black-Scholes option pricing model was developed for use in
estimating the fair value of short-term traded options that have no
vesting restrictions and are fully transferable. In addition,
option valuation models require the input of highly subjective
assumptions including expected stock price volatility. Because the
Company’s common stock warrants have characteristics
significantly different from those of traded options and because
changes in the subjective input assumptions can materially affect
the fair value estimate, in management’s opinion the existing
models do not necessarily provide a reliable single measure of the
fair value of its common stock warrants. During the years ended
December 31, 2018 and 2017 there were no warrants
granted with an exercise price below the fair value of the
underlying stock at the grant date.
The
weighted average fair value of warrants granted with exercise
prices at the current fair value of the underlying stock was
approximately $0.08122 per warrant granted during the year ended
December 31, 2018.
Premier
Biomedical, Inc.
Notes to Financial
Statements
The
following is a summary of activity of outstanding common stock
warrants:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance,
December 31, 2016
|
122,760
|
$61.0175
|
|
Warrants
granted
|
163,000
|
1.25
|
|
Warrants
exercised
|
(28,000)
|
(0.0025)
|
|
Balance, December
31, 2017
|
257,760
|
$29.85
|
|
Warrants
granted
|
3,656,000
|
0.09
|
|
Warrants
exercised
|
(12,000)
|
(0.0025)
|
|
Balance, December
31, 2018
|
3,901,760
|
$2.05
|
|
|
|
|
|
Exercisable,
December 31, 2018
|
3,901,760
|
$2.05
|
Note 13 – Income Taxes
The
Company accounts for income taxes under FASB ASC 740-10, which
requires use of the liability method. FASB ASC 740-10-25 provides
that deferred tax assets and liabilities are recorded based on the
differences between the tax bases of assets and liabilities and
their carrying amounts for financial reporting purposes, referred
to as temporary differences.
For the
years ended December 31, 2018 and 2017, the Company incurred a net
operating loss and, accordingly, no provision for income taxes has
been recorded. In addition, no benefit for income taxes has been
recorded due to the uncertainty of the realization of any tax
assets. At December 31, 2018 and December 31, 2017, the
Company had approximately $5,277,000 and $4,860,000 of federal net
operating losses, respectively. The net operating loss carry
forwards, if not utilized, will begin to expire in
2031.
The
components of the Company’s deferred tax asset are as
follows:
|
|
|
|
|
|
|
|
|
Deferred tax
assets:
|
|
|
|
Net operating loss
carry forwards
|
$1,108,170
|
$1,701,000
|
|
|
|
|
|
Net deferred tax
assets before valuation allowance
|
$1,108,170
|
$1,701,000
|
|
Less: Valuation
allowance
|
(1,108,170)
|
(1,701,000)
|
|
Net deferred tax
assets
|
$-
|
$-
|
Based
on the available objective evidence, including the Company’s
history of losses, management believes it is more likely than not
that the net deferred tax assets will not be fully realizable.
Accordingly, the Company provided for a full valuation allowance
against its net deferred tax assets at December 31, 2018
and 2017, respectively.
A
reconciliation between the amounts of income tax benefit determined
by applying the applicable U.S. and State statutory income tax rate
to pre-tax loss is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal and state
statutory rate
|
21%
|
35%
|
|
Change in valuation
allowance on deferred tax assets
|
(21%)
|
(35%)
|
In
accordance with FASB ASC 740, the Company has evaluated its tax
positions and determined there are no uncertain tax
positions.
Premier
Biomedical, Inc.
Notes to Financial
Statements
Note 14 – Subsequent Events
Convertible Debt Financing
On
March 26, 2019, the Company received proceeds of $68,000 in
exchange for a 10% interest bearing; unsecured convertible
promissory note maturing on March 26, 2020 (“First Power
Up Lending Note”). The note is convertible 180 days from the
date of the note at 61% of the average of the two lowest closing
bid prices of the Common Stock in the twenty (20) Trading Days
prior to the Conversion Date.
Common Stock Issuances for Debt Conversions
On
March 22, 2019, the Company issued 386,000 shares of common stock
pursuant to the conversion of $6,369, consisting of $2,136 of
principal and $4,233 of interest, from the Second Diamond Rock
Note. The note was converted in accordance with the conversion
terms; therefore, no gain or loss has been recognized.
On
March 6, 2019, the Company issued 370,000 shares of common stock
pursuant to the conversion of $5,739 of principal from the Second
Diamond Rock Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
February 26, 2019, the Company issued 349,463 shares of common
stock pursuant to the conversion of $6,500 of principal from the
First SEG-RedaShex Note. The note was converted in accordance with
the conversion terms; therefore, no gain or loss has been
recognized.
On
February 26, 2019, the Company issued 340,000 shares of common
stock pursuant to the conversion of $5,273 of principal from the
Second Diamond Rock Note. The note was converted in accordance with
the conversion terms; therefore, no gain or loss has been
recognized.
On
February 12, 2019, the Company issued 346,200 shares of common
stock pursuant to the conversion of $6,924 of principal from the
Second Diamond Rock Note. The note was converted in accordance with
the conversion terms; therefore, no gain or loss has been
recognized.
On
February 1, 2019, the Company issued 315,000 shares of common stock
pursuant to the conversion of $7,875 of principal from the Second
Diamond Rock Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
January 23, 2019, the Company issued 260,000 shares of common stock
pursuant to the conversion of $6,513 of principal from the Second
Diamond Rock Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
January 11, 2019, the Company issued 280,000 shares of common stock
pursuant to the conversion of $5,597 of principal from the Second
Diamond Rock Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
On
January 2, 2019, the Company issued 281,385 shares of common stock
pursuant to the conversion of $6,500 of principal from the First
SEG-RedaShex Note. The note was converted in accordance with the
conversion terms; therefore, no gain or loss has been
recognized.
Common Stock Issuances on Subscriptions Payable
On
January 1, 2019, the Company issued 276,960 shares to DiamondRock, LLC for the conversion of
$5,345 of debt on December 31, 2018.
PART II – INFORMATION NOT REQUIRED IN PROSPECTUS
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
We will
pay all expenses in connection with the registration and sale of
the common stock by the selling shareholder, who is an underwriter
in connection with their offering of shares. The estimated expenses
of issuance and distribution are set forth below:
|
Registration
Fees
|
Approximately
|
$13
|
|
Transfer Agent
Fees
|
Approximately
|
1,000
|
|
Costs of Printing
and Engraving
|
Approximately
|
1,000
|
|
Legal
Fees
|
Approximately
|
15,000
|
|
Accounting and
Audit Fees
|
Approximately
|
5,000
|
|
Total
|
|
$22,013
|
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Article
9 of our Articles of Incorporation provides that, the personal
liability of the directors of the corporation is hereby eliminated
to the fullest extent permitted by paragraph 1 of Section 78.037 of
the General Corporation Law of the State of Nevada, as the same may
be amended and supplemented. Paragraph 1 of Section 78.037 states
that the articles of incorporation of a Nevada corporation may
contain any provision, not contrary to the laws of the State of
Nevada, for the management of the business and for the conduct of
the affairs of the corporation.
Article
10 of our Articles of Incorporation provides that, the corporation
shall, to the fullest extent permitted by Section 78.751 of the
General Corporation Law of the State of Nevada, as the same may be
amended and supplemented, indemnify any and all persons whom it
shall have power to indemnify under said section from and against
any and all expenses, liabilities, or other matters referred to in
or covered by said section. Section 78.751 states that the articles
of incorporation of a Nevada corporation may provide that the
expenses of officers and directors incurred in defending a civil or
criminal action, suit or proceeding must be paid by the corporation
as they are incurred and in advance of the final disposition. It
further states that indemnification does not exclude any other
rights that an officer or director may have pursuant to the
articles, bylaws, shareholders agreement or otherwise, and that it
continues for a person who has ceased to be a director, officer, or
employee of the company.
Article
V of our Bylaws further addresses indemnification, including
procedures for indemnification claims. Indemnification applies to
any person that is made a party to, or threatened to be made a
party to, any action, suit or proceeding, whether civil, criminal,
administrative or investigative, by reason of the fact that he or
she was an officer or director of the company.
Insofar
as indemnification for liabilities arising under the Securities Act
of 1933 (the “Act”) may be permitted to directors,
officers and controlling persons of the registrant pursuant to the
foregoing provisions, or otherwise, the registrant has been advised
that in the opinion of the Securities and Exchange Commission such
indemnification is against public policy as expressed in the Act
and is, therefore, unenforceable.
RECENT SALES OF UNREGISTERED SECURITIES
The
following is a list of unregistered sales of equity securities
issued by the Company from January 1, 2015 through the date
hereof.
Common Stock
2015
LG Capital Funding, LLC
On
January 30, 2015, we entered into a Securities Purchase Agreement
with LG Capital Funding, LLC (“LG Capital”), pursuant
to which we sold to LG Capital a 8% Convertible Promissory Note in
the original principal amount of $82,687.00 (the “LG
Note”). The LG Note has a maturity date of January 29, 2016,
and is convertible after 180 days into our common stock at the higher of (i) $0.25 cents per
share or (ii) 70% of the average of the two (2) lowest closing bid
prices of our common stock for the fifteen (15) trading days prior
to receipt of a conversion notice from LG Capital. The shares of
common stock issuable upon conversion of the LG Note will be
restricted securities as defined in Rule 144 promulgated under the
Securities Act of 1933. The LG Note can be prepaid by us at a
premium as follows: (a) between 1 and 30 days after issuance
– 115% of the principal amount; (b) between 31 and 60 days
after issuance – 121% of the principal amount; (c) between 61
and 90 days after issuance – 126% of the principal amount;
(d) between 91 and 120 days after issuance – 132% of the
principal amount; (e) between 121 and 150 days after issuance
– 138% of the principal amount; and (f) between 151 and 180
days after issuance – 140% of the principal amount. There is
no right to pre-payment after 180 days. The purchase and sale of
the LG Note closed on January 30, 2015, the date that the purchase
price was delivered to us. The issuance of the LG Note was exempt
from the registration requirements of the Securities Act of 1933
pursuant to Section 4(a)(2) of the Securities Act of 1933. The
purchaser was an accredited and sophisticated investor, familiar
with our operations, and there was no solicitation.
Adar Bays, LLC
On
February 24, 2015, we entered into a Securities Purchase Agreement
with Adar Bays, LLC (“Adar Bays”), pursuant to which we
sold to Adar Bays an 8% Convertible Promissory Note in the original
principal amount of $44,100.00 (the “Adar Note”). The
Adar Note has a maturity date of February 24, 2016, and is
convertible after 180 days into our common stock at the higher of (i) $0. 25 cents per
share or (ii) 70% of the average of the two (2) lowest closing
prices of our common stock for the fifteen (15) trading days prior
to receipt of a conversion notice from Adar Bays. The shares of
common stock issuable upon conversion of the Adar Note will be
restricted securities as defined in Rule 144 promulgated under the
Securities Act of 1933. The Adar Note can be prepaid by us at a
premium as follows: (a) between 1 and 30 days after issuance
– 115% of the principal amount; (b) between 31 and 60 days
after issuance – 121% of the principal amount; (c) between 61
and 90 days after issuance – 127% of the principal amount;
(d) between 91 and 120 days after issuance – 133% of the
principal amount; (e) between 121 and 150 days after issuance
– 139% of the principal amount; and (f) between 151 and 180
days after issuance – 140% of the principal amount. There is
no right to pre-payment after 180 days. The purchase and sale of
the Adar Note closed on March 2, 2015, the date that the purchase
price was delivered to us. The issuance of the Adar Note was exempt
from the registration requirements of the Securities Act of 1933
pursuant to Section 4(a)(2) of the Securities Act of 1933. The
purchaser was an accredited and sophisticated investor, familiar
with our operations, and there was no solicitation.
Services
On
March 20, 2015, we issued a warrant to acquire two thousand (2,000)
shares of our common stock in exchange for services rendered. The
warrant is exercisable for five (5) years at $0.20 per share, but
only vests to the extent that the closing bid price of our common
stock reaches the following levels over three consecutive trading
days:
●
500 warrants will
vest at $50 per share;
●
500 warrants will
vest at $75 per share;
●
500 warrants will
vest at $100 per share; and
●
500 warrants will
vest at $125 per share.
The
issuance of the warrants was exempt from the registration
requirements of the Securities Act of 1933 pursuant to Section
4(a)(2) of the Securities Act of 1933. The purchaser was a
sophisticated investor, familiar with our operations, and there was
no solicitation.
On May
30, 2015, we issued a warrant to acquire up to two thousand (2,000)
shares of our common stock to Ryan Fields, one of the members of
our Scientific Advisory Board, in exchange for services related
thereto. The warrant is exercisable for seven (7) years at $62.50
and shall vest in its entirety on December 1, 2015, subject to the
condition that Mr. Fields is still a member of our Scientific
Advisory Board at that time. If Mr. Fields ceases to be a member of
our Scientific Advisory Board for any reason prior to December 1,
2015, the warrant shall terminate immediately. The issuance of the
warrant was exempt from the registration requirements of the
Securities Act of 1933 pursuant to Section 4(a)(2) of the
Securities Act of 1933. The purchaser was a sophisticated investor,
familiar with our operations, and there was no
solicitation.
JMJ Financial
On
September 2, 2015, we entered into a Convertible Promissory Note
with JMJ Financial (“JMJ”) in the original principal
amount of up to $250,000 (the “JMJ Note”). The initial
amount of funding under the JMJ Note was $50,000. The JMJ Note has
a maturity date of two (2) years from each funding and is
convertible at any time by the holder into our common stock at 60%
of the lowest trade price in the twenty five (25) trading days
previous to the conversion, with a floor of $0.025 per share. The
shares of common stock issuable upon conversion of the JMJ Note
will be restricted securities as defined in Rule 144 promulgated
under the Securities Act of 1933. Any funding under the JMJ Note
can be prepaid by us within ninety (90) days without a premium and
without interest. After ninety (90) days, a one-time interest
charge of twelve percent (12%) is applied, and the JMJ Note may not
be prepaid without the holder’s consent. The issuance of
the JMJ Note was exempt from the registration requirements of
the Securities Act of 1933 pursuant to Section 4(a)(2) of the
Securities Act of 1933. The purchaser was an accredited and
sophisticated investor, familiar with our operations, and there was
no solicitation.
Warrant Exercise
On
September 10, 2015, we issued 4,000 shares of common stock,
restricted in accordance with Rule 144, to Mitchell Felder, one of
our directors, upon the exercise of warrants at $0.0025 per share.
The issuance was exempt from the registration requirements of the
Securities Act of 1933 pursuant to Section 4(a)(2) of the
Securities Act of 1933. The purchaser was an accredited and
sophisticated investor, familiar with our operations, and there was
no solicitation.
Advisory Board Warrants
On
September 10, 2015, we issued warrants to purchase two thousand
(2,000) shares of our common stock to a new member of our
Scientific Advisory Board. The exercise price of the warrants is
$25 per share. The warrants are vested immediately. The issuance
was exempt from registration pursuant to Section 4(a)(2) of the
Securities Act of 1933, the investors are sophisticated and
familiar with our operations, and there was no solicitation in
connection with the issuance.
Vis Vires Group, Inc.
On
September 8, 2015, we entered into a Securities Purchase Agreement
with Vis Vires Group, Inc., pursuant to which we sold to Vires a 8%
Convertible Promissory Note in the original principal amount of
Forty Eight Thousand Dollars ($48,000.00) (the “Vires
Note”). The Vires Note has a maturity date of June 8, 2016,
and is convertible after 180 days into our common stock at the
greater of (i) the Variable Conversion Price and (ii) the Fixed
Conversion Price. The “Variable Conversion Price” shall
mean 58% multiplied by the Market Price (representing a discount
rate of 42%). “Market Price” means the average of the
lowest three (3) Trading Prices for the Common Stock during the ten
(10) Trading Day period ending on the latest complete Trading Day
prior to the Conversion Date. “Trading Price” means the
closing bid price on the applicable day. The “Fixed
Conversion Price” shall mean $0.0025. The shares of common
stock issuable upon conversion of the Vires Note will be restricted
securities as defined in Rule 144 promulgated under the Securities
Act of 1933. The Vires Note can be prepaid by us at a premium as
follows: (a) between 0 and 30 days after issuance – 110% of
the principal amount and any accrued and unpaid interest; (b)
between 31 and 60 days after issuance – 115% of the principal
amount and any accrued and unpaid interest; (c) between 61 and 90
days after issuance – 120% of the principal amount and any
accrued and unpaid interest; (d) between 91 and 120 days after
issuance – 125% of the principal amount and any accrued and
unpaid interest; (e) between 121 and 150 days after issuance
– 130% of the principal amount and any accrued and unpaid
interest; and (f) between 151 and 180 days after issuance –
135% of the principal amount and any accrued and unpaid interest.
The purchase and sale of the Vires Note closed on September 21,
2015, the date that the purchase price was delivered to us. The
issuance of the Vires Note was exempt from the registration
requirements of the Securities Act of 1933 pursuant to Section
4(a)(2) thereof. The purchaser was an accredited and sophisticated
investor, familiar with our operations, and there was no
solicitation.
Warrant Exercise
On
October 1, 2015, we issued 12,000 shares of common stock,
restricted in accordance with Rule 144, to Mitchell Felder, one of
our directors, upon the exercise of warrants at $0.0025 per share.
The issuance was exempt from the registration requirements of the
Securities Act of 1933 pursuant to Section 4(a)(2) of the
Securities Act of 1933. The purchaser was an accredited and
sophisticated investor, familiar with our operations, and there was
no solicitation.
Effective
as of October 21, 2015, we issued warrants to the following
officers and directors, which will allow them to purchase shares of
our common stock in the amounts indicated: William Hartman (4,000
shares); Mitchell Felder (4,000 shares), Heidi Carl (3,000 shares),
John Borza (2,400 shares), Richard Najarian (800 shares), and Jay
Rosen (800 shares). We also issued warrants to purchase a total of
720 shares of our common stock to six members of our Scientific
Advisory Board. The exercise price of the foregoing warrants is
$12.50 per share. One half of the shares underlying each of the
respective warrants vest on June 15, 2016, with the balance vesting
on December 15, 2016. In order for the warrants to vest on each of
the foregoing dates, however, the holders must be serving in the
same capacity on behalf of the Company as he or she was serving on
October 21, 2015. The issuance of the warrants was fully approved
by our Board of Directors on October 21, 2015, the date a fully
executed resolution authorizing the issuance was delivered to us.
The issuances were exempt from registration pursuant to Section
4(a)(2) of the Securities Act of 1933 and Rule 506 of Regulation D
promulgated thereunder, the investors are sophisticated and
familiar with our operations, and there was no solicitation in
connection with the issuance.
2016
Redwood Management, LLC
On
December 28, 2015, we entered into a Securities Purchase Agreement
with Redwood Management, LLC (“Redwood”), pursuant to
which we agreed to sell, and Redwood agreed to purchase, One
Million Six Hundred Thousand Dollars ($1,600,000) in 10%
Convertible Promissory Notes. On February 26, 2016, and again on
March 7, 2016, the Securities Purchase Agreement was amended, and
the total amount of funding to which Redwood is obligated was
reduced to $525,000. The notes have an original issue discount of
five percent (5%). The first note was issued on December 28, 2015
in the face amount of One Hundred Fifty Seven Thousand Five Hundred
Dollars ($157,500), to Redwood Management, LLC. The second note was
issued on January 8, 2016, in the face amount of One Hundred Thirty
One Thousand Two Hundred Fifty Dollars ($131,250), to Redwood Fund
III Ltd. The third note was issued on February 22, 2016, in the
face amount of Seventy Eight Thousand Seven Hundred Fifty Dollars
($78,750), to Redwood Management, LLC. The fourth note was issued
on March 7, 2016, in the face amount of Seventy Eight Thousand
Seven Hundred Fifty Dollars ($78,750), to Redwood Management, LLC.
The fifth and final note was issued on March 11, 2016, in the face
amount of One Hundred Five Thousand Dollars ($105,000). The
maturity date of each note is nine (9) months after its issuance.
Each note will be convertible after ninety (90) days into our
common stock at a conversion price equal to 60% of the lowest
traded price of the Common Stock in the fifteen (15) Trading Days
prior to the Conversion Date. The shares of common stock issuable
upon conversion of the notes will be restricted securities as
defined in Rule 144 promulgated under the Securities Act of 1933.
The notes can be prepaid by us at any time upon ten (10) days
written notice to Redwood for a cash
amount equal to the sum of the then outstanding principal amount of
the note and interest multiplied by 130%. Pursuant to a
Registration Rights Agreement, we agreed to register the shares
underlying conversion of the notes. The purchase and sale of the
initial note closed on December 28, 2015, the date that the
purchase price was delivered to us.
On
October 10, 2016, we entered into an Exchange Agreement by and
between the Company and Redwood. Pursuant to the Exchange
Agreement, we exchanged the Typenex Note for the Warrant. The
Typenex Note has a principal amount of $300,000, an interest rate
of 10% and matures on October 10, 2017, unless earlier converted
into shares of our common stock. The Typenex Note may be converted
to common stock at any time after January 8, 2017. The conversion
price for the Typenex Note is equal to 60% of the lowest traded
price of our common stock in the 15 trading days prior to the
conversion date. If any shares of our common stock are sold at an
effective price per share that is lower than the conversion price,
the conversion price will be adjusted down to match the lower
price. We have instructed our transfer agent to reserve 600,000
shares of our common stock for conversions pursuant to the Typenex
Note. This reserve will stay in place until the Typenex Note and
any interest due thereunder is satisfied in full.
Pursuant to the
Purchase Agreement, we have issued the following shares of common
stock on the dates indicated and in the amounts shown to
Redwood:
|
Date
|
|
|
|
7/21/2016
|
$29,000
|
16,112
|
|
8/9/2016
|
$39,520
|
20,800
|
|
11/8/2016
|
$25,000
|
24,391
|
|
11/22/2016
|
$25,000
|
25,511
|
|
12/13/2016
|
$25,000
|
25,000
|
|
1/4/2017
|
$10,323
|
12,589
|
|
Totals
|
$153,843
|
124,403
|
The
issuances to Redwood listed above were exempt from registration
pursuant to Section 4(a)(2) of the Securities Act of 1933, the
investor is sophisticated and familiar with our operations, and
there was no solicitation in connection with the sale.
Preferred Stock
On
January 2, 2016, two of our officers and directors, William A.
Hartman and Mitchell Felder, each exercised warrants to acquire one
million (1,000,000) shares of Series A Convertible Preferred Stock
each. Each share of Series A Convertible Preferred Stock is
convertible, at the election of the holder thereof, into one (1)
share of our common stock, and has one hundred (100) votes per
share. We issued the warrants on June 21, 2010 and they had an
exercise price of $0.001 per share.
The
Preferred Stock also contains protective provisions as
follows:
The
Company may not take any of the following actions without the
approval of a majority of the holders of the outstanding Series A
Convertible Preferred Stock: (i) effect a sale of all or
substantially all of the Company’s assets or which results in
the holders of the Company’s capital stock prior to the
transaction owning less than fifty percent (50%) of the voting
power of the Company’s capital stock after the transaction,
(ii) alter or change the rights, preferences, or privileges of
the Series A Convertible Preferred Stock, (iii) increase or
decrease the number of authorized shares of Series A Convertible
Preferred Stock, (iv) authorize the issuance of securities having a
preference over or on par with the Series A Convertible Preferred
Stock, or (v) effectuate a forward or reverse stock split or
dividend of the Company’s common stock.
Common Stock
On
February 10, 2016, we issued 12,000 shares of our common stock,
restricted in accordance with Rule 144, to a third-party for
services rendered in connection with our recent financing
transactions. The issuance was exempt from registration pursuant to
Section 4(a)(2) of the Securities Act of 1933, the investor was
sophisticated and familiar with our operations, and there was no
solicitation in connection with the sale.
On
February 12, 2016, we issued 2,400shares of our common stock,
restricted in accordance with Rule 144, to a third-party for
services rendered in connection with our recent financing
transactions. The issuance was exempt from registration pursuant to
Section 4(a)(2) of the Securities Act of 1933, the investor was
sophisticated and familiar with our operations, and there was no
solicitation in connection with the sale.
On
March 28, 2016, upon the resignation of Richard Najarian as one of
the members of our Board of Directors, we issued to him 2,400
shares of common stock in settlement of an unpaid expense
reimbursement. The issuance was exempt from registration pursuant
to Section 4(a)(2) of the Securities Act of 1933, the investor was
sophisticated and familiar with our operations, and there was no
solicitation in connection with the sale.
The Note
On May
27, 2016, we issued a 10% convertible promissory note to Redwood
pursuant to the Note (as describe above). The issuance of the Note
was exempt from registration pursuant to Section 4(a)(2) of the
Securities Act of 1933 and Rule 506 of Regulation D promulgated
thereunder. The purchaser was an accredited and sophisticated
investor, familiar with our operations, and there was no
solicitation.
Warrant Exercise
On
August 19, 2016, we issued 16,000 shares of common stock,
restricted in accordance with Rule 144, to each of William Hartman
and Mitchell Felder, officers and directors of the Company, upon
the exercise of warrants at $0.0025 per share. The issuance was
exempt from the registration requirements of the Securities Act of
1933 pursuant to Section 4(a)(2) of the Securities Act of 1933. The
purchaser was an accredited and sophisticated investor, familiar
with our operations, and there was no solicitation.
On
December 20, 2016, we issued 24,000 shares of common stock,
restricted in accordance with Rule 144, to each of William Hartman
and Mitchell Felder, officers and directors of the Company, upon
the exercise of warrants at $0.0025 per share. The issuance was
exempt from the registration requirements of the Securities Act of
1933 pursuant to Section 4(a)(2) of the Securities Act of 1933. The
purchaser was an accredited and sophisticated investor, familiar
with our operations, and there was no solicitation.
2017
Private Placement of Stock, Warrants and Convertible
Notes
On
March 30, 2017, we entered into Securities Purchase Agreements by
and between the Company and each of The Special Equities Group,
LLC, RDW Capital, LLC and DiamondRock, LLC to sell our common stock
and warrants at a fixed price. On March 30, 2017, we closed the
first sale under these agreements, receiving $100,000 from each
investor in exchange for 53,334 shares of our common stock,
warrants to purchase up to 53,334 shares of our common stock at an
exercise price of $7.50 (“Series A Warrants”) and warrants
to purchase up to 53,334 shares or our common stock at an exercise
price of $12.50 (“Series B
Warrants” and together with the Series A Warrants, the
“Warrants”). In
the aggregate, we received $300,000 in exchange for 160,002 shares
of our common stock, 160,002 Series A Warrants and 160,002 Series B
Warrants.
On May
30, 2017, we completed the second sale under the Securities
Purchase Agreement, dated March 30, 2017. We sold 40,405 shares of
our common stock, 40,405 Series A Warrants and 40,405 Series B
Warrants to each of The Special Equities Group, LLC, RDW Capital,
LLC and DiamondRock, LLC for $50,000, receiving an aggregate of
$150,000 in exchange for 121,215 shares of our common stock,
121,215 Series A Warrants and 121,215 Series B
Warrants.
On
August 8, 2017, we entered into Exchange Agreements with The
Special Equities Group, LLC, RDW Capital, LLC and DiamondRock, LLC
whereby we exchanged convertible notes with an aggregate face value
of $150,000 for the following securities previously issued pursuant
to the Securities Purchase Agreements, dated March 30, 2017:
121,215 shares of common stock, 160,002 Series A Warrants and
160,002 Series B Warrants. The convertible notes are convertible
into shares of our common stock, bear interest at 8% and matured on
November 30, 2017. The notes were convertible at 50% of the lowest
traded price of our common stock in the fifteen (15) Trading Days
prior to the Conversion Date.
On
October 30, 2017, we completed the third sale under the Securities
Purchase Agreements, dated March 30, 2017, as amended. We sold a
convertible promissory note for $50,000 to each of The Special
Equities Group, LLC, RDW Capital, LLC and DiamondRock, LLC, an
aggregate of $150,000. The convertible notes are convertible into
shares of our common stock, bear interest at 8% and mature on
January 31, 2018. The notes are convertible at 50% of the lowest
traded price of our common stock in the fifteen (15) Trading Days
prior to the Conversion Date.
On
November 22, 2017, we issued 28,000 shares of common stock,
restricted in accordance with Rule 144, to William Hartman, an
officer and director of the Company, upon the exercise of warrants
at $0.0025 per share.
The
above transactions were exempt from the registration requirements
of the Securities Act of 1933 pursuant to Section 4(a)(2) of the
Securities Act of 1933. The purchasers were accredited and
sophisticated investors, familiar with our operations, and there
was no solicitation.
2018
On
March 1, 2018, we entered into a Securities Purchase Agreement (the
“Purchase
Agreement”) by and between the Company and the Selling
Shareholders to sell Convertible Promissory Notes (each a
“Note” and
collectively, the “Notes”). Pursuant to the Purchase
Agreement, the Selling Shareholders will pay an aggregate of
$300,000 for the Notes in three tranches or closings.
The
Selling Shareholders purchased Notes at the signing of the Purchase
Agreement for an aggregate amount of $60,000. The Selling
Shareholders purchased a second tranche of Notes for an aggregate
of $60,000 on April 24, 2018 after we filed a registration
statement to cover the Selling Shareholders’ shares of common
stock issuable upon conversion of the Notes. Within five trading
days of the registration statement being declared effective, the
Selling Shareholders will buy additional Notes for an aggregate of
$180,000 (the “Third
Tranche”).
2019
Securities Purchase Agreement and Convertible Note
On March 28, 2019, we entered into a Securities
Purchase Agreement (the “Purchase
Agreement”) by and
between the Company and Power Up Lending Group Ltd. (the
“Purchaser”) to sell a Convertible Promissory Note
(“Note”). The Purchaser purchased the Note at the
signing of the Purchase Agreement for an aggregate amount of
$68,000. The Note has a maturity date of March 26, 2020, an
interest rate of 10% and a default interest rate of 22%. The Note
is convertible into our common stock at a conversion price equal to
61% of the average of the lowest two (2) trading prices during the
last twenty (20) trading days prior to the conversion
date.
The
sale of the Note pursuant to the Purchase Agreement and in the
transaction described above was offered and sold in reliance on an
exemption from registration pursuant to Section 4(a)(2) of the
Securities Act of 1933, as amended, and Rule 506 of Regulation D.
The Purchaser has represented that it is an accredited investor, as
defined in Regulation D, and has acquired the securities for
investment purposes only and not with a view to, or for sale in
connection with, any distribution thereof. The securities were not
issued through any general solicitation or
advertisement.
On
March 27, 2019, we entered into a Securities Purchase Agreement
(the “Purchase Agreement”) by and between the Company
and Crown Bridge Partners, LLC (the “Purchaser”) to
sell Convertible Promissory Notes (each a “Note”) in
the principal amount of up to $154,500, with a purchase price of up
to $141,000. The Purchaser purchased the first Note on April 17,
2019 for an aggregate amount of $47,000. The Note has a maturity
date of twelve (12) months from each funding date, or April 17,
2020 with respect to the first Note. Each Note has an interest rate
of 12% and a default interest rate of 15%. The Note is convertible
into our common stock at a conversion price equal to 60% of the
lowest trading price during the last twenty (20) trading days prior
to the conversion date.
On
April 23, 2019, we entered into a Securities Purchase Agreement
(the “Purchase Agreement”) by and between the Company
and Power Up Lending Group Ltd. (the “Purchaser”) to
sell a Convertible Promissory Note (“Note”). The
Purchaser purchased the Note at the signing of the Purchase
Agreement for an aggregate amount of $38,000. The Note has a
maturity date of April 23, 2020, an interest rate of 10% and a
default interest rate of 22%. The Note is convertible into our
common stock at a conversion price equal to 61% of the average of
the lowest two (2) trading prices during the last twenty (20)
trading days prior to the conversion date.
On
June 7, 2019, we entered into a Securities Purchase Agreement (the
“Purchase Agreement”) by and between the Company and
Power Up Lending Group Ltd. (the “Purchaser”) to sell a
Convertible Promissory Note (“Note”). The Purchaser
purchased the Note at the signing of the Purchase Agreement for an
aggregate amount of $38,000. The Note has a maturity date of June
7, 2020, an interest rate of 10% and a default interest rate of
22%. The Note is convertible into our common stock at a conversion
price equal to 61% of the average of the lowest two (2) trading
prices during the last twenty (20) trading days prior to the
conversion date. We closed the sale of the Note on June 12,
2019.
Securities Purchase Agreement and Convertible Note - Crown Bridge
Partners, LLC
On
March 27, 2019, we entered into a Securities Purchase Agreement
(the “Purchase Agreement”) by and between the Company
and Crown Bridge Partners, LLC (the “Purchaser”) to
sell Convertible Promissory Notes (each a “Note”) in
the principal amount of up to $154,500, with a purchase price of up
to $141,000. The Purchaser purchased the first Note on April 17,
2019 for an aggregate amount of $47,000 (the “First
Tranche”). The Note has a maturity date of twelve (12) months
from each funding date, or April 17, 2020 with respect to the first
Note. Each Note has an interest rate of 12% and a default interest
rate of 15%. The Note is convertible into our common stock at a
conversion price equal to 60% of the lowest trading price during
the last twenty (20) trading days prior to the conversion date. The
above stated transaction was reported in Premier’s April 17,
2019 Form 8-K.
On July
8, 2019, pursuant to the Securities Purchase Agreement, the
Purchaser purchased the second Note for an aggregate amount of
$32,900 (the “Second Tranche”). The Note has a maturity
date of twelve (12) months from each funding date, or July 8, 2020
with respect to the second Note. Each Note has an interest rate of
12% and a default interest rate of 15%. The Note is convertible
into our common stock at a conversion price equal to 60% of the
lowest trading price during the last twenty (20) trading days prior
to the conversion date.
On
September 13, 2019, pursuant to the Purchase Agreement, the
Purchaser purchased the third Note for an aggregate amount of
$23,500 (the “Third Tranche”). The third Note has a
maturity date of September 13, 2020.
We must
reserve shares of our authorized common stock equal to four times
the number of shares issuable upon full conversion of the Note,
initially 26,580,645 shares. The Note can be prepaid by us at any
time for a cash amount equal to the sum of the then outstanding
principal amount of the note and interest multiplied by a
prepayment percentage that ranges from as low as 125% to as high as
150%, depending on when we prepay the Note.
The
Note limits the Purchaser to beneficial ownership of our common
stock of no more than 4.99%. The Purchaser has the right to receive
any dividend or distribution of assets as if the Note had been
fully converted on the applicable record date. The Purchase
Agreement and Note also contain customary representations and
warranties made by the Company and by the Purchaser.
The
sale of the Note pursuant to the Purchase Agreement was offered and
sold in reliance on an exemption from registration pursuant to
Section 4(a)(2) of the Securities Act of 1933, as amended, and Rule
506 of Regulation D. The Purchaser has represented that it is an
accredited investor, as defined in Regulation D, and has acquired
the securities for investment purposes only and not with a view to,
or for sale in connection with, any distribution thereof. The
securities were not issued through any general solicitation or
advertisement.
Securities Purchase Agreement and Convertible Note – Power Up
Lending Group Ltd.
On
August 2, 2019, we entered into a Securities Purchase Agreement
(the “Purchase Agreement”) by and between the Company
and Power Up Lending Group Ltd. (the “Purchaser”) to
sell a Convertible Promissory Note (“Note”). The
Purchaser purchased the Note at the signing of the Purchase
Agreement for an aggregate amount of $38,000. The Note has a
maturity date of August 2, 2020, an interest rate of 10% and a
default interest rate of 22%. The Note is convertible into our
common stock at a conversion price equal to 61% of the average of
the lowest two (2) trading prices during the last twenty (20)
trading days prior to the conversion date. We closed the sale of
the Note on August 7,
2019.
On
August 15, 2019, we entered into a Securities Purchase Agreement
(the “Purchase Agreement”) by and between the Company
and Power Up Lending Group Ltd. (the “Purchaser”) to
sell a Convertible Promissory Note (“Note”). The
Purchaser purchased the Note at the signing of the Purchase
Agreement for an aggregate amount of $43,000. The Note has a
maturity date of August 15, 2020, an interest rate of 10% and a
default interest rate of 22%. The Note is convertible into our
common stock at a conversion price equal to 61% of the average of
the lowest two (2) trading prices during the last twenty (20)
trading days prior to the conversion date. We closed the sale of
the Note on August 16,
2019.
We must
reserve shares of our authorized common stock equal to four times
the number of shares issuable upon full conversion of the Note,
initially 40,849,234. The Note can be prepaid by us at any time
upon three (3) days written notice to the Purchaser for a cash
amount equal to the sum of the then outstanding principal amount of
the note and interest multiplied by a prepayment percentage that
ranges from as low as 115% to as high as 140%, depending on when we
prepay the Note.
The
Note limits the Purchaser to beneficial ownership of our common
stock of no more than 4.99%. The Purchaser has the right to receive
any dividend or distribution of assets as if the Note had been
fully converted on the applicable record date. The Purchase
Agreement and Note also contain customary representations and
warranties made by the Company and by the Purchaser.
The
sale of the Note pursuant to the Purchase Agreement was offered and
sold in reliance on an exemption from registration pursuant to
Section 4(a)(2) of the Securities Act of 1933, as amended, and Rule
506 of Regulation D. The Purchaser has represented that it is an
accredited investor, as defined in Regulation D, and has acquired
the securities for investment purposes only and not with a view to,
or for sale in connection with, any distribution thereof. The
securities were not issued through any general solicitation or
advertisement.
EXHIBITS
(a)
The exhibits listed
on the Exhibit Index at the end of this Registration Statement are
incorporated herein and filed as part of this registration
statement.
(b)
Financial Statement
Schedules.
No
financial statement schedules have been provided because the
information is not required or is shown either in the financial
statements or the notes thereto.
Undertakings
A.
Insofar as
indemnification for liabilities arising under the Securities Act of
1933 may be permitted to our directors, officers and controlling
persons pursuant to the foregoing provisions, or otherwise, we have
been advised that in the opinion of the Securities and Exchange
Commission such indemnification is against public policy as
expressed in the Securities Act of 1933and is, therefore,
unenforceable. In the event that a claim for indemnification
against such liabilities (other than the payment by us of expenses
incurred or paid by our director, officer or controlling person in
the successful defense of any action, suit or proceeding) is
asserted by such director, officer or controlling person in
connection with the securities being registered, we will, unless in
the opinion of our counsel the matter has been settled by
controlling precedent, submit to a court of appropriate
jurisdiction the question whether such indemnification by it is
against public policy as expressed in the Securities Act of 1933
and will be governed by the final adjudication of such
issue.
B.
The undersigned
registrant hereby undertakes:
(1)
To file, during any
period in which offers or sales are being made, a post-effective
amendment to this registration statement:
(a)
To include any
prospectus required by Section 10(a)(3) of the Securities Act of
1933;
(b)
To reflect in the
prospectus any facts or events arising after the effective date of
the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate,
represent a fundamental change in the information set forth in the
registration statement. Notwithstanding the foregoing, any increase
or decrease in volume of securities offered (if the total dollar
value of securities offered would not exceed that which was
registered) and any deviation from the low or high end of the
estimated maximum offering range may be reflected in the form of
prospectus filed with the Commission pursuant to Rule 424(b)
(Section 230.424(b) of Regulation S-K) if, in the aggregate, the
changes in volume and price represent no more than a 20% change in
the maximum aggregate offering price set forth in the
“Calculation of Registration Fee” table in the
effective registration statement; and
(c)
To include any
material information with respect to the plan of distribution not
previously disclosed in the registration statement or any material
change to such information in the registration
statement.
(2)
That, for the
purpose of determining any liability under the Securities Act of
1933, each such post-effective amendment shall be deemed to be a
new registration statement relating to the securities offered
therein, and the offering of the securities at that time shall be
deemed to be the initial bona fide offering thereof.
(3)
To remove from
registration by means of a post-effective amendment any of the
securities being registered which remain unsold at the termination
of the offering.
(4)
That, for the
purpose of determining liability under the Securities Act of 1933
to any purchaser:
(i) If
the registrant is relying on Rule 430B (§230.430B of this
chapter):
(a)
Each prospectus
filed by the registrant pursuant to Rule 424(b)(3)
(§230.424(b)(3) of this chapter) shall be deemed to be part of
the registration statement as of the date the filed prospectus was
deemed part of and included in the registration statement;
and
(b)
Each prospectus
required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7)
(§230.424(b)(2), (b)(5), or (b)(7) of this chapter) as part of
a registration statement in reliance on Rule 430B relating to an
offering made pursuant to Rule 415(a)(1)(i), (vii), or (x)
(§230.415(a)(1)(i), (vii), or (x) of this chapter) for the
purpose of providing the information required by section 10(a) of
the Securities Act of 1933 shall be deemed to be part of and
included in the registration statement as of the earlier of the
date such form of prospectus is first used after effectiveness or
the date of the first contract of sale of securities in the
offering described in the prospectus. As provided in Rule 430B, for
liability purposes of the issuer and any personthat is at that date
an underwriter, such date shall be deemed to be a new effective
date of the registration statement relating to the securities in
the registration statement to which that prospectus relates, and
the offering of such securities at that time shall be deemed to be
the initial bona fide
offering thereof. Provided,
however, that no statement made in a registration statement
or prospectus that is part of the registration statement or made in
a document incorporated or deemed incorporated by reference into
the registration statement or prospectus that is part of the
registration statement will, as to a purchaser with a time of
contract of sale prior to such effective date, supersede or modify
any statement that was made in the registration statement or
prospectus that was part of the registration statement or made in
any such document immediately prior to such effective date;
or
(ii)
If the registrant
is subject to Rule 430C (§230.430C of this chapter), each
prospectus filed pursuant to Rule 424(b) as part of a registration
statement relating to an offering, other than registration
statements relying on Rule 430B or other than prospectuses filed in
reliance on Rule 430A (§230.430A of this chapter), shall be
deemed to be part of and included in the registration statement as
of the date it is first used after effectiveness. Provided, however, that no statement
made in a registration statement or prospectus that is part of the
registration statement or made in a document incorporated or deemed
incorporated by reference into the registration statement or
prospectus that is part of the registration statement will, as to a
purchaser with a time of contract of sale prior to such first use,
supersede or modify any statement that was made in the registration
statement or prospectus that was part of the registration statement
or made in any such document immediately prior to such date of
first use.
(5)
That, for the
purpose of determining liability of the registrant under the
Securities Act of 1933 to any purchaser in the initial distribution
of the securities:
(i)
The
undersigned registrant undertakes that in a primary offering of
securities of the undersigned registrant pursuant to this
registration statement, regardless of the underwriting method used
to sell the securities to the purchaser, if the securities are
offered or sold to such purchaser by means of any of the following
communications, the undersigned registrant will be a seller to the
purchaser and will be considered to offer or sell such securities
to such purchaser:
(a)
Any
preliminary prospectus or prospectus of the undersigned registrant
relating to the offering required to be filed pursuant to Rule 424
(§230.424 of this chapter);
(b)
Any
free writing prospectus relating to the offering prepared by or on
behalf of the undersigned registrant or used or referred to by the
undersigned registrant;
(c)
The
portion of any other free writing prospectus relating to the
offering containing material information about the undersigned
registrant or its securities provided by or on behalf of the
undersigned registrant; and
(d)
Any
other communication that is an offer in the offering made by the
undersigned registrant to the purchaser.
(6)
The
undersigned registrant hereby undertakes that:
(i)
For purposes of determining any liability under the Securities
Act of 1933, the information omitted from the form of prospectus
filed as part of this registration statement in reliance upon Rule
430A and contained in a form of prospectus filed by the registrant
pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities
Act shall be deemed to be part of this registration statement as of
the time it was declared effective.
(ii)
For
the purpose of determining any liability under the Securities Act
of 1933, each post-effective amendment that contains a form of
prospectus shall be deemed to be a new registration statement
relating to the securities offered therein, and the offering of
such securities at that time shall be deemed to be the initial bona
fide offering thereof.
SIGNATURES
Pursuant to the
requirements of the Securities Act of 1933, the registrant has duly
caused this registration statement to be signed on its behalf by
the undersigned, thereunto duly authorized, in the City of Jackson
Center, State of Pennsylvania.
|
|
Premier Biomedical, Inc.
|
|
|
|
|
Dated: December 9, 2019
|
/s/ William A. Hartman
|
|
|
By: William A.
Hartman
|
|
|
Its: Chief Executive
Officer
|
|
|
|
|
Dated: December 9, 2019
|
/s/ Heidi H. Carl
|
|
|
By: Heidi H.
Carl
|
|
|
Its: Chief
Financial Officer, Treasurer and Principal
Accounting Officer
|
Pursuant to the
requirements of the Securities Act of 1933, this registration
statement has been signed by the following persons in the
capacities and on the dates indicated.
|
Dated: December 9, 2019
|
/s/ William A. Hartman
|
|
|
Name:
William A. Hartman
Title:
Chief Executive Officer, President and Director
|
|
|
|
|
Dated: December 9, 2019
|
/s/ Mitchell S. Felder
|
|
|
Name:
Mitchell S. Felder
Title:
Chairman of the Board and Director
|
|
|
|
|
Dated: December 9, 2019
|
/s/ Heidi H. Carl
|
|
|
Name:
Heidi H. Carl
Title:
Chief Financial Officer, Secretary and Director
|
|
|
|
|
Dated: December 9, 2019
|
/s/ John S. Borza
|
|
|
Name:
John S. Borza
Title:
Director
|
|
|
|
|
Dated: December 9, 2019
|
/s/ Patricio Reyes
|
|
|
Name:
Patricio Reyes
Title:
Director
|
|
|
|
|
Dated: December 9, 2019
|
/s Jay
Rosen
|
|
|
Name:
Jay Rosen
Title:
Director
|
|
|
|
|
Dated: December 9, 2019
|
/s/
John Pauly
|
|
|
Name:
John Pauly
Title:
Director
|
EXHIBIT INDEX
|
Exhibit
No.
|
|
Description
of Exhibits
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit
No.
|
|
Description
of Exhibits
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit No.
|
|
Description
|
|
10.31
(18)
|
|
Securities Purchase Agreement with Power Up Lending Group Ltd.
dated March 26, 2019
|
|
|
|
|
|
10.32
(18)
|
|
Convertible Promissory Note with Power Up Lending Group Ltd. dated
March 26, 2019
|
|
|
|
|
|
10.33
(19)
|
|
Securities Purchase Agreement with Crown Bridge Partners, LLC
entered into on March 27, 2019
|
|
|
|
|
|
10.34
(19)
|
|
Convertible Promissory Note with Crown Bridge Partners, LLC entered
into on March 27, 2019
|
|
|
|
|
|
10.35
(19)
|
|
Securities Purchase Agreement with Power Up Lending Group, Ltd.
entered into on April 23, 2019
|
|
|
|
|
|
10.36
(19)
|
|
Convertible Promissory Note with Power Up Lending Group, Ltd.
entered into on April 23, 2019
|
|
|
|
|
|
10.37
(19)
|
|
Securities Purchase Agreement with Power Up Lending Group, Ltd.
entered into on June 7, 2019
|
|
|
|
|
|
10.38
(19)
|
|
Convertible Promissory Note with Power Up Lending Group, Ltd.
entered into on June 7, 2019
|
|
|
|
|
|
|
|
|
(1)
Incorporated by
reference from our Registration Statement on Form S-1 dated June
13, 2011, filed with the Commission on June 14, 2011.
(2)
Incorporated by
reference from our Current Report on Form 8-K dated February 9,
2016, filed with the Commission on February 10, 2016.
(3)
Incorporated by
reference from our Registration Statement on Form S-1/A dated and
filed with the Commission on October 4, 2011.
(4)
Incorporated by
reference from our Current Report on Form 8-K filed with the
Commission on May 14, 2012.
(5)
Incorporated by
reference from our Current Report on Form 8-K filed with the
Commission on October 10, 2012.
(6)
Incorporated by
reference from our Annual Report on Form 10-K filed with the
Commission on April 1, 2013.
(7)
Incorporated by
reference from our Current Report on Form 8-K dated February 20,
2013, filed with the Commission on February 27, 2013.
(8)
Incorporated by
reference from our Current Report on Form 8-K dated and filed with
the Commission on June 12, 2013.
(9)
Incorporated by
reference from our Current Report on Form 8-K dated March 16, 2015,
filed with the Commission on March 18, 2015.
(10)
Incorporated by
reference from our Quarterly Report on Form 10-Q dated May 14,
2015, filed with the Commission on May 15, 2015.
(11)
Incorporated by
reference from our Current Report on Form 8-K dated June 19, 2015,
filed with the Commission on June 23, 2015.
(12)
Incorporated by
reference from our Quarterly Report on Form 10-Q dated and filed
with the Commission on August 14, 2015.
(13)
Incorporated by
reference from our Registration Statement on Form S-1 (File No.
333-218250) filed with the Commission on May 26, 2017.
(14)
Incorporated by
reference from our Quarterly Report on Form 10-Q dated and filed
with the Commission on August 21, 2017.
(15)
Incorporated by
reference from our Registration Statement on Form S-1/A dated and
filed with the Commission on October 16, 2017.
(16)
Incorporated by
reference from our Annual Report dated and filed with the
Commission on April 12, 2018.
(17)
Incorporated by
reference from our Current Report on Form 8-K dated and filed with
the Commission on November 29, 2018.
(18)
Incorporated by
reference from our Quarterly Report dated and filed with the
Commission on May 15, 2019.
(19)
Incorporated by
reference from our Quarterly Report dated and filed with the
Commission on August 14, 2019.
Premier Biomedical (PK) (USOTC:BIEI)
Historical Stock Chart
From Mar 2024 to Apr 2024
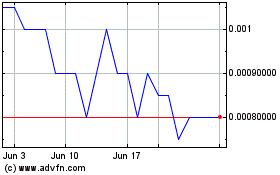
Premier Biomedical (PK) (USOTC:BIEI)
Historical Stock Chart
From Apr 2023 to Apr 2024