Filed
Pursuant to Rule 424(b)(5)
Registration
No. 333-213280
PROSPECTUS
SUPPLEMENT
(to
Prospectus dated August 31, 2016)
810,000
American Depositary Shares Representing 40,500,000 Ordinary Shares

Medigus
Ltd.
We
are offering 810,000 American Depositary Shares, or ADSs, representing 40,500,000 of our ordinary shares, par value NIS 0.10 per
share, at a price of $2.00 per ADS, pursuant to this prospectus supplement and the accompanying prospectus. Each ADS represents
50 ordinary shares. See “Description of American Depositary Shares” and “Description of Ordinary Shares”
in the accompanying prospectus for more information.
In
a concurrent private placement, we are selling to the investors warrants to purchase up to 405,000 ADSs, representing 20,250,000
of our ordinary shares, par value NIS 0.10 per share, at an initial exercise price of $2.25 per ADS, or the Warrants. The Warrants,
the ADSs issuable upon the exercise of the Warrants and the ordinary shares represented by such ADSs are being offered pursuant
to an exemption from registration provided in Section 4(a)(2) under the Securities Act of 1933, as amended, or the Securities
Act, and Rule 506(b) promulgated thereunder, and they are not being offered pursuant to this prospectus supplement and the accompanying
prospectus.
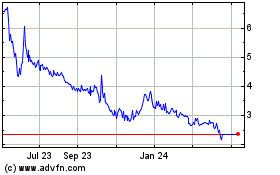
Our ADSs trade on the
Nasdaq Capital Market, or the NASDAQ, under the symbol “MDGS”. On November 22, 2017, the last reported sale
price of our ADSs on NASDAQ was $2.23 per ADS. Our ordinary shares currently trade on the Tel Aviv Stock Exchange Ltd., or
TASE, under the symbol “MDGS.” On November 23, 2017, the last reported sale price of our ordinary shares on the
TASE was NIS 0.152, or $0.043 per share (based on the exchange rate reported by the Bank of Israel on such date).
On October 20, 2017, the aggregate market value of our ordinary shares held by non-affiliates was $7,171,181,
based on 151,285,784 ordinary shares outstanding (equivalent to 3,025,715 ADSs) and a per ADS price of $2.39 based on the closing
sale price of our ADSs on NASDAQ on October 20, 2017. We have sold an aggregate of approximately $763,244 of our securities pursuant
to General Instruction I.B.5 on Form F-3 during the prior 12 calendar month period that ends on and includes the date of this prospectus.
You
should carefully read this prospectus supplement and the accompanying prospectus (including all of the information incorporated
by reference therein) before you invest. Investing in our securities involves a high degree of risk. Before buying any securities,
you should read the discussion of material risks of investing in our securities in the section entitled “Risk Factors”
beginning on page S-10 of this prospectus supplement.
|
|
|
Per ADS
|
|
|
Total
|
|
|
Offering price
|
|
$
|
2.00
|
|
|
$
|
1,620,000
|
|
|
Placement agent fee
(1)
|
|
$
|
0.14
|
|
|
$
|
113,400
|
|
|
Proceeds, before expenses, to us
|
|
$
|
1.86
|
|
|
$
|
1,506,600
|
|
|
(1)
|
In
addition to the placement agent cash fee listed in the table above, we have agreed to reimburse the placement agent for certain
of its expenses with respect to this offering and issue warrants to purchase our ADSs as described under “Plan of Distribution”
on page S-39 of this prospectus supplement.
|
Delivery
of the ADSs is expected to be made on or about November 28, 2017.
None
of the United States Securities and Exchange Commission, the Israeli Securities Authority, any state securities commission or
any other regulatory body, has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus
supplement or the accompanying prospectus. Any representation to the contrary is a criminal offense.
Prospectus
Supplement dated November 24, 2017
Table
Of Contents
ABOUT
THIS PROSPECTUS SUPPLEMENT
You
should rely only on the information incorporated by reference or provided in this prospectus supplement and the accompanying prospectus,
or to which we have referred you. We have not authorized anyone to provide you with different information. If anyone provides
you with different or inconsistent information, you should not rely on it. This prospectus supplement and the accompanying prospectus
do not constitute an offer to sell, or a solicitation of an offer to purchase, the securities offered by this prospectus supplement
and the accompanying prospectus in any jurisdiction where it is unlawful to make such offer or solicitation. You should not assume
that the information contained in this prospectus supplement or the accompanying prospectus, or any document incorporated by reference
in this prospectus supplement or the accompanying prospectus, is accurate as of any date other than the date on the front cover
of the applicable document. Neither the delivery of this prospectus supplement nor any distribution of securities pursuant to
this prospectus supplement shall, under any circumstances, create any implication that there has been no change in the information
set forth or incorporated by reference into this prospectus supplement or in our affairs since the date of this prospectus supplement.
Our business, financial condition, results of operations and prospects may have changed since that date.
A registration statement
on Form F-3 (File No. 333-213280) utilizing a shelf registration process relating to the securities described in this prospectus
supplement was initially filed with the Securities and Exchange Commission, or the SEC, on August 24, 2016, and was declared effective
on August 31, 2016. Under this shelf registration process, of which this offering is a part, we may, from time to time, sell up
to an aggregate of $20 million of our securities. We have sold an aggregate of $763,244 of our securities under this shelf registration
process during the prior 12 calendar month period that ends on and includes the date of this prospectus supplement. This document
comprises two parts. The first part is this prospectus supplement, which describes the specific terms of this offering and also
adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference herein. The
second part, the accompanying prospectus, gives more general information, some of which may not apply to this offering. Generally,
when we refer to this prospectus supplement, we are referring to both parts of this document combined. If the description of the
offering varies between this prospectus supplement and the accompanying prospectus or the documents incorporated herein by reference
filed prior to the date of this prospectus supplement, you should rely on the information contained in this prospectus supplement.
However, if any statement in one of these documents is inconsistent with a statement in another document having a later date —
for example, a document incorporated by reference in the accompanying prospectus — the statement in the document having the
later date modifies or supersedes the earlier statement.
Before
purchasing any securities, you should carefully read both this prospectus supplement and the accompanying prospectus, together
with the additional information described under the headings, “Where You Can Find More Information” and “Incorporation
of Information by Reference,” on page S-42 of this prospectus supplement.
Unless
the context otherwise requires, all references to “Medigus,” “we,” “us,” “our,”
the “Company” and similar designations refer to Medigus Ltd. The term “NIS” refers to New Israeli Shekels,
the lawful currency of the State of Israel, the terms “dollar”, “US$” or “$” refer to U.S.
dollars, the lawful currency of the United States. Our functional and presentation currency is the U.S. dollar. Foreign currency
transactions in currencies other than the U.S. dollar are translated in this prospectus supplement into U.S. dollars using exchange
rates in effect at the date of the transactions.
We
further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any
document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including,
in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation,
warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made.
Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state
of our affairs.
We
are offering to sell, and seeking offers to buy, ADSs representing our ordinary shares only in jurisdictions where offers and
sales are permitted. The distribution of this prospectus supplement and the accompanying prospectus and the offering of the ADSs
in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus
supplement and the accompanying prospectus must inform themselves about, and observe any restrictions relating to, the offering
of the ADSs and the distribution of this prospectus supplement and the accompanying prospectus outside the United States. This
prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection with, an offer to sell,
or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying prospectus by
any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus supplement, the accompanying prospectus, and the information incorporated by reference herein and therein may include
forward looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other U.S. Federal securities
laws. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance
or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking
statements. In some cases, you can identify forward-looking statements by terms including “anticipates”, “believes”,
“could”, “estimates”, “expects”, “intends”, “may”, “plans”,
“potential”, “predicts”, “projects”, “should”, “will”, “would”,
and similar expressions intended to identify forward-looking statements. Forward-looking statements reflect our current views
with respect to future events and are based on assumptions and subject to risks and uncertainties. In addition, certain sections
of this prospectus supplement, the accompanying prospectus, and the information incorporated by reference herein contain information
obtained from independent industry and other sources that we have not independently verified. You should not put undue reliance
on any forward-looking statements. Unless we are required to do so under U.S. federal securities laws or other applicable laws,
we do not intend to update or revise any forward-looking statements.
Our
ability to predict our operating results or the effects of various events on our operating results is inherently uncertain. Therefore,
we caution you to consider carefully the matters described under the caption “Risk Factors” on page S-10 of this
prospectus supplement, and certain other matters discussed in this prospectus supplement, the accompanying prospectus, and the
information incorporated by reference herein and therein, and other publicly available sources. Such factors and many other factors
beyond our control could cause our actual results, performance or achievements to be materially different from any future results,
performance or achievements that may be expressed or implied by the forward-looking statements.
Factors
that could cause our actual results to differ materially from those expressed or implied in such forward-looking statements include,
but are not limited to:
|
|
●
|
the
overall global economic environment;
|
|
|
●
|
insufficient
coverage or reimbursement from medical insurers;
|
|
|
●
|
the
impact of competition and new technologies;
|
|
|
●
|
general
market, political, reimbursement and economic conditions in the countries in which we
operate;
|
|
|
●
|
our
ability to continue as a going concern;
|
|
|
●
|
projected
capital expenditures and liquidity;
|
|
|
●
|
changes
in our strategy;
|
|
|
●
|
government
regulations and approvals;
|
|
|
●
|
changes
in customers’ budgeting priorities;
|
|
|
●
|
litigation
and regulatory proceedings;
|
|
|
●
|
those
factors referred to the “Risk Factors” found on page S-10 of this prospectus
supplement; and
|
|
|
●
|
those
factors referred to in “Item 3. Key Information – D. Risk Factors,”
“Item 4. Information on the Company,” and “Item 5. Operating
and Financial Review and Prospects” in our Annual Report on Form 20-F for the year
ended December 31, 2016.
|
We
caution you to carefully consider these risks and not to place undue reliance on our forward-looking statements. Except as required
by law, we assume no responsibility for updating any forward-looking statements.
PROSPECTUS
SUPPLEMENT SUMMARY
This
summary highlights selected information about us, this offering and information contained in greater detail elsewhere in this
prospectus supplement, the accompanying prospectus, and in the documents incorporated by reference. This summary is not complete
and does not contain all of the information that you should consider before investing in our ADSs. You should carefully read and
consider this entire prospectus supplement, the accompanying prospectus and the documents, including financial statements and
related notes, and information incorporated by reference into this prospectus supplement, including the financial statements and
“Risk Factors” starting on page S-10 of this prospectus supplement, before making an investment decision. If you invest
in our securities, you are assuming a high degree of risk.
Overview
Who
We Are
We
are a medical device company dedicated to the development, manufacturing and marketing of surgical endostaplers and direct vision
systems for minimally invasive medical procedures or other commercial use. Our expertise is in the development, production and
marketing of innovative surgical devices with direct visualization capabilities for the treatment of Gastroesophageal Reflux Disease,
or GERD, a common ailment, which is predominantly treated by medical therapy (e.g. proton pump inhibitors) or in chronic cases,
conventional open or laparoscopic surgery. Our FDA-cleared and CE-marked endosurgical system, known as the MUSE
TM
(Medigus
Ultrasonic Surgical Endostapler) system, enables minimally-invasive and incisionless procedures for the treatment of GERD by reconstruction
of the esophageal valve via the mouth and esophagus, eliminating the need for surgery in eligible patients. We believe that this
procedure offers a safe, effective and economical alternative to the current modes of GERD treatment for certain GERD patients,
and has the ability to provide results which are equivalent to those of standard surgical procedures while reducing pain and trauma,
minimizing hospital stays, and delivering economic value to hospitals and payors.
The
key elements of the MUSE
TM
system include a single-use endostapler containing several sophisticated innovative technologies
such as flexible stapling technology, a miniature camera and ultrasound sensor, as well as a control console offering a video
image transmitted from the tip of the endostapler.
In
addition to the MUSE
TM
system for the treatment of GERD, we have developed miniaturized video cameras for use in various
medical procedures as well as specialized industrial applications.
Prevalence
of GERD
GERD,
is a worldwide disorder, with evidence suggesting an increase in GERD disease prevalence since 1995. The sample size weighted
mean for the GERD population in the United States and Europe is 19.8% and 15.2% respectively. In the United States alone, over
49 million adults are affected by the disease, with over 29 million adults suffering daily GERD symptoms. Proton pump inhibitors,
or PPIs, are a class of effective and generally safe medication to treat GERD, but not everyone who experiences heartburn needs
a PPI. Several PPIs have been widely advertised to consumers and heavily promoted by physicians. This has led to an overuse of
the drug. PPIs are the third highest selling class of drugs in the U.S. and Nexium has the second highest retail sales among all
drugs at $4.8 billion in 2008. This figure does not include sales of other brands of PPIs.
After
being swallowed, food descends through the esophagus to the stomach, which contains acids and enzymes intended to digest and break
down food. GERD is caused by the defective operation of the lower esophageal sphincter, or LES, a valve, which controls the flow
of ingested food from the esophagus into the stomach. While eating and between eating periods, a properly operating LES prevents
stomach contents from entering the esophagus. Among GERD sufferers, the valve opens spontaneously or is unable to close properly.
This results in acidic stomach contents rising into the esophagus, causing irritation, acid reflux and heartburn, as well as other
potentially dangerous conditions.
Beyond
painful symptoms, GERD may also increase sufferers’ susceptibility to cancer. Whereas the stomach is lined by the “gastric
mucosal barrier” which allows acidic material to be contained harmlessly, the surface of the esophagus consists of flat,
thin cells called squamous cells, which are not resistant to acid. Repeated episodes of acid reflux can cause inflammation
of the esophagus, a condition called esophagitis. The flat cells lining the esophagus can also undergo genetic changes due to
exposure to acid, causing these cells to resemble those found in the stomach lining, a condition known as Barrett’s Esophagus.
Barrett’s
Esophagus is a complication of GERD and predisposes patients to esophageal adenocarcinoma, a tumor that has increased in incidence
more than 7-fold over the past several decades. Studies have shown that people exhibiting Barrett’s Esophagus have a higher
risk of developing cancer of the esophagus. Studies have also shown, that compared to patients not exhibiting GERD symptoms, patients
exhibiting weekly symptoms of GERD have a five times higher probability for developing esophageal cancer while patients exhibiting
daily symptoms of GERD have a seven times higher probability for developing esophageal cancer. The most common risk factors for
cancer in Barrett esophagus patients include chronic GERD, hiatal hernia, advanced age, male sex, white race, cigarette smoking
and obesity.
Treatment
of GERD
Treatment
of GERD involves a stepwise approach. The goals are to control symptoms, to heal esophagitis and to prevent recurrent esophagitis.
The treatment is based on lifestyle modification and control of gastric acid through medical treatment (antacids, PPI’s,
H2 blockers or other reflux inhibitors) or antireflux surgery. Mild GERD may be defined as intermittent reflux symptoms that can
be managed with lifestyle changes or over-the-counter medications. Moderate to severe GERD represents more chronic symptoms that
may require stronger drugs, long term medication or surgical intervention.
Drug
treatment - Proton pump inhibitors (PPI)
For
moderate to severe GERD, physicians usually prescribe proton pump inhibiting drugs, or PPI. This class of drugs reduces acid production
by the stomach, and thereby relieves the patients of their symptoms. Drugs of this class are among the most commonly prescribed
medications in the world. There are several brands on the market, best known are Prilosec (omeprazole), Prevacid (lansoprazole)
and Nexium (esomeprazole). Certain PPI drugs are available over the counter in the United States and in other countries, but the
over the counter dosage may be inadequate to control GERD symptoms, except in mild cases.
While
PPI drugs effectively reduce the severity and frequency of GERD symptoms, they have a number of drawbacks:
a)
In approximately 30% of patients, symptom control is incomplete;
b)
The drugs do not treat the disease, they only control its manifestations, therefore must be taken for life at a dosage which requires
prescription. Accumulated costs may be substantial; and
c)
Long term use is associated with a number of serious adverse effects. In particular, they increase the risk of osteoporosis and
fractures of the hip, wrist and spine. The FDA had issued a warning on this effect as well as warnings against other untoward
effects on absorption of other essential minerals, which may lead to chronic kidney disease, irregular heartbeat, diarrhea and
increased flatulence.
Interventional
treatment
The
most common operation for GERD is called a surgical fundoplication, a procedure that prevents reflux by wrapping or attaching
the upper part of the stomach around the lower esophagus and securing it with sutures. Due to the presence of the wrap or attachment,
increasing pressure in the stomach compresses the portion of the esophagus which is wrapped or attached by the stomach, and prevents
acidic gastric content from flowing up into the esophagus. Today, the operation is usually performed laparoscopically: instead
of a single large incision into the chest or abdomen, four or five smaller incisions are made in the abdomen, and the operator
uses a number of specially designed tools to operate under video control.
The
operation does not completely eliminate the use of PPI, and up to approximately 60% still use some in long term follow up. Nevertheless,
the dose is usually lower – in the over the counter range - and the response rate is excellent. Since the majority of patients
referred to surgeons are incomplete responders, or require a high dose of PPI, the patients are generally satisfied with the operation,
and the overall costs of treatment are lower in the long run.
In
spite of the clinical outcome of surgery, relatively few patients undergo surgery. We estimate that large numbers of patients
who are candidates for operative treatment are either not referred by their treating physician or decline it. We believe that
many patients decline to undergo operations to avoid even minute scars or violation of the abdominal cavity.
Given
the current environment in which the vast majority of GERD sufferers in North America and Europe must choose between long-term
pharmaceutical therapy and surgery, leading to what is known in our industry as the “treatment gap”, we believe there
is a demand for a minimally-invasive, incision-less procedure which treats the root cause of the disease. We believe that the
MUSE
TM
system is positioned to fill this need.
Our
system achieves the general physiological result of surgical fundoplication, by inserting the MUSE
TM
endostapler
through the mouth and the esophagus, and stapling the top of the stomach to the side of the esophagus. The endostapler contains
a video camera and stapling system. Staples have long been used in surgical procedures in place of sutures, and we believe that
they are at least as reliable and potentially more durable. Our endostapler uses standard surgical staples.
First
line therapy for GERD includes a combination of lifestyle modifications and medical therapy, or PPIs. Unfortunately, 25% to 42%
of patients with GERD do not respond to an initial 4-8-week treatment of PPI. In those who do respond to therapy, the effectiveness
of PPI treatment decreases over time. Antireflux surgery controls acid reflux and treats an incompetent lower esophageal sphincter,
while also improving patient quality of life in the long term. Thus, PPI therapy and lifestyle modifications are frequently eliminated.
Despite
the effectiveness of surgery, it is invasive, requires hospitalization, and carries the risk of short and long-term complications,
including dysphagia, diarrhea, and gas bloat syndrome Thus, endoscopic therapies that mimic the mechanism through which surgery
works and can reduce surgical morbidity have gained popularity for the treatment of GERD.
The
market for medical devices, including the market for endoscopic therapies, is very broad, with an increasing demand for new less
invasive alternatives to the existing surgical procedures for the treatment of various diseases. This increasing need for minimally
invasive and incision-less treatments, such as endoscopy-based procedures, are also augmented by the increase in the average age
of global population. In 2000, the worldwide population of persons aged more than 65 years was an estimated 420 million. During
2000-2030, the worldwide population aged more than 65 years is projected to increase by approximately 550 million to 973 million.
This increase in age will potentially lead to increased health-care costs and may have dramatic consequences for public health
and the health care financing and delivery systems significant patient benefits and cost savings.
Endoscopy
is a minimally invasive method of performing investigative, diagnostic and therapeutic medical procedures, employing an endoscope,
which allows real-time visual observation of the patient’s internal organs during the procedure. Endoscopic procedures are
most commonly performed through natural orifices, including via the mouth, to avoid incisions. Because of the accessibility of
the digestive tract through the mouth, the endoscopy field is largely focused on disorders of the gastrointestinal tract such
as disorders of the colon, esophagus, stomach and duodenum.
Endoscopes
are commonly composed of a flexible tube with a camera installed at its tip. Endoscopes often include “working channels”
through which catheters or other endoscopic tools or devices may be inserted directly into the patient’s digestive system.
The primary advantage of endoscopy is the elimination of incisions to the patient’s body during a medical procedure. We
believe that this is safer, prevents most post-operative pain and facilitates faster recuperation. Patient perception or preference
is important as well. The perception of endoscopy procedures as being safer, and less painful than, corresponding surgical procedures
may have the effect of minimizing patient fears.
Endoscopic
procedures generally involve less recovery time and patient discomfort than conventional open or laparoscopic surgery. These procedures
are also typically performed in the outpatient hospital setting as opposed to an inpatient setting. Typically, outpatient procedures
cost the hospital or the insurer less money since there is no overnight stay in the hospital.
Our
Solution
The MUSE ™ System
Our
primary product, the MUSE
TM
single use system for transoral funduplication, is an innovative device for the incisionless
treatment of GERD. The MUSE
TM
technology is based on our proprietary platform technology, experience and know-how.
While at present substantially all of our revenue is derived from the miniature video camera and related equipment, our strategy
is focused on the development and promotion of the MUSE
TM
System, which we therefore refer to as our ‘primary
product’.
Transoral
means the procedure is performed through the mouth, rather than through incisions in the abdomen. The MUSE
TM
system for transoral fundoplication was previously known as the SRS
TM
Endoscopic Stapling System. We rebranded
to the MUSE
TM
system following the launch of the most recent generation product. The MUSE
TM
system is used
to perform a procedure as an alternative to a surgical fundoplication. The MUSE
TM
offers an endoscopic, incisionless
alternative to surgery. A single surgeon or gastroenterologist can perform the MUSE
TM
procedure in a trans-oral way,
unlike in a laparoscopic fundoplication which requires incisions.
The
system consists of the MUSE
TM
console controller, the MUSE
TM
endostapler and several accessories, including
an overtube, irrigation bottle, tubing supplies and staple cartridges. The MUSE
TM
endostapler incorporates a video
camera, a flexible surgical stapler and an ultrasonic guidance system, that is used to measure the distance between the anvil
and the cartridge of the stapler, to ensure their proper alignment and tissue thickness. The device also contains an alignment
pin, which is used for initial positioning of the anvil against the cartridge, two anvil screws, which are used to reduce the
thickness of the tissue that needs to be stapled and to fix the position of the anvil and the MUSE
TM
endostapler during
stapling. The system allows the operator to staple the fundus of the stomach to the esophagus, in two or more locations, typically
around the circumference, thereby creating a fundoplication, without any incisions.
The
clearance by the FDA, or ‘Indications for Use’, of the MUSE
TM
System is “for endoscopic
placement of surgical staples in the soft tissue of the esophagus and stomach in order to create anterior partial
fundoplication for treatment of symptomatic chronic Gastro-Esophageal Reflux Disease in
patients who require and respond to pharmacological therapy”. As such, the FDA clearance covers the use by an
operator of the MUSE
TM
endostapler as described in the above paragraph. In addition, in the pivotal study
that was presented to the FDA in order to gain clearance,only patients who were currently taking GERD medications(i.e.
pharmacological therapy) were allowed in the study. In addition, all patients had to have a significant decrease in
their symptoms when they were taking medication compared to when they were off the medication. As such, the FDA
clearance included the indication that MUSE
TM
is intended for patients who require and respond to pharmacological
therapy. The MUSE
TM
System indication does not restrict its use with respect to GERD severity from a
regulatory point of view. However, clinicians typically only consider interventional treatment options for moderate to severe
GERD. Therefore, it is reasonable to expect the MUSE
TM
System would be primarily used to treat moderate and
severe GERD in practice. The system has received 510(k) marketing clearance from the FDA in the United States, as well as a
CE mark in Europe and a license from Health Canada. It is also cleared for use in Turkey and in Israel.
Clinical
Studies
The
original FDA submission for the MUSE
TM
System included short-term (6 month) results from a multi-center clinical trial.
The trial was conducted in support of the 510(k) marketing clearance submission for the system and pursuant to an FDA-issued Investigational
Device Exemption (IDE).
Enrollment
was completed in November 2010. A total of 72 patients were enrolled and 69 were treated with the MUSE
TM
system during
the study. A manuscript detailing the results of this study was published in Surgical Endoscopy and is currently available online.
Publication in the hardcopy of the journal was in the January 2015 issue.
The
primary objective of the study was to assess the safety and efficacy of the MUSE
TM
system in the treatment of subjects
with GERD. The primary efficacy endpoint was at least a 50% improvement in the GERD-HRQL (Health Related Quality of Life) scores
in 53% of the subjects. HRQL is the standard assessment of how an individual’s well-being may be affected over time by a disease.
Secondary efficacy assessments included PPI intake, esophageal acid exposure during a 24-hour period and anatomical changes. The
follow-up period was set at six months following each procedure.
The
primary endpoint was met in that 73% of subjects exhibited at least a 50% reduction in HRQL at six months. In addition, 85% of
subjects reduced their PPI intake by at least 50%, with 65% of subjects eliminating PPI use completely at six months.
FDA
marketing clearance for our system was granted in May 2012 following the original FDA submission. Subsequent improvements to the
system included improvements to the camera, illumination and alignment mechanisms, the addition of an electronic stapling motor,
and condensing two control consoles into a single unit. FDA clearance for the modified system was obtained in March 2014. The
modified system has also obtained a CE mark in Europe and a license from Health Canada and was approved in Turkey and Israel.
In
May 2013, we received five years of follow-up results for a precursor IRB (Institutional Review Board) approved pilot study of
the system conducted in 2007 at Deenanath Mangeshkar Hospital and Research Center in the city of Pune, India. The results of this
follow-up study were published in the peer review journal Surgical Endoscopy in March 2015. As noted in the journal article,
the five-year results are similar to the results obtained from subjects who received-laparoscopic procedures for GERD in the same
period. Each year, eleven of the thirteen patients were reached (although not always the same eleven). All thirteen patients had
at least a four year follow-up. Throughout the follow up period, GERD-HRQL scores were normal in all but one patient. All patients
indicated that they would agree to do the procedure again. Out of the initial thirteen patients, seven (54%) had eliminated PPI
and another three (23%) reduced PPI use by 50% or more. It should be emphasized that for this trial patients were selected with
GERD severity at a higher than average level (moderate to severe), a fact which may indicate an even greater outcome of the effect
of the system in an average GERD level patient population.
In
November 2015 a follow-up study conducted in the United States looked at evaluating the long-term clinical outcome of 37 patients
who received GERD treatment with the MUSE
TM
device in the multi-center study mentioned above was concluded. Efficacy
and safety data were analyzed up to four years post-procedure. No new complications have been reported in such long-term analysis.
The proportions of patients who remained off daily PPI were 83.8% (31/37) at six months, and 69.4% (25/36) at 4 years post-procedure.
GERD-HRQL scores off PPI were significantly decreased following six months and four years post-procedure. The authors concluded
that the MUSE
TM
stapling device appeared to be safe and effective in improving symptom scores as well as reducing PPI
use in patients with GERD. These results appeared to be equal to or better than those of the other devices for endoluminal GERD
therapy.
In
February 2017, we received an approval to start a multi-center MUSE
TM
clinical study in China after the China Food
and Drug Administration, or CFDA, reviewed the complete submission package. In addition, each study location received approval
from their ethics committee and agreements were put in place.. Under Principal Investigator, Yunsheng Yang, Director of Gastroenterology
Department Clinical center at 301 Hospital and Chairman of Chinese Society of Gastroenterology, The General Hospital of People’s
Liberation Army in Beijing, the clinical study will include approximately 62 patients, will take place at five centers across
China: The General Hospital of People’s Liberation Army, Renji Hospital of Shanghai, Shanghai General Hospital, Peking University
Third Hospital and Navy General Hospital.
Procedures
started in March 2017 and are expected to carry on through 2018. We expect the results to be reported back to the CFDA in 2019
as part of the final CFDA submission for clearance to sell MUSE
TM
in China.
Miniature
Video Cameras
By
definition all endoscopes must include vision apparatus to facilitate the operator’s view of the internal organs of the
patient. In the past, fiber optics were utilized for this purpose, and have been gradually replaced with electronic video systems
offering higher resolution and higher-quality images. We have developed several models of miniaturized digital video cameras and
video processing equipment, for use in medical endoscopy products as well as industrial uses. Our cameras range between 3.45mm
to 0.99 mm in diameter, and are based on single-use Complementary Metal Oxide Semiconductor, or CMOS, image sensors. In some cases,
our cameras are relatively inexpensive, allowing them to be used in single-use devices.
Our
miniature cameras are intended for use in medical applications in which it has not yet been feasible to use miniature video cameras,
and may be integrated into devices developed by the company, or by third parties who source the camera from us. We expect that
the growing demand for single-use medical devices will increase demand for the CMOS cameras in particular, in fields such as gastroenterology,
orthopedics, gynecology, ears nose throat, urology, cardio-vascular, and other fields in which diagnostic and surgical procedures
may be performed endoscopically. Small-diameter video cameras permit not only smaller camera-based endoscopes which are able to
penetrate previously inaccessible organs or visualize them in improved image quality, but also allows for the addition of working
channels and other features in the valuable space freed by the reduction in camera size.
Our
most advanced camera is a prototype CMOS-based camera measuring only 0.99 mm in diameter transmitting 45,000 pixels in HDMI format,
which we believe to be the smallest video camera ever produced. This camera is based on “through-silicon-via” technology
whereby the electronics pass vertically through the sensor, permitting smaller diameter devices. This prototype camera will not
be commercially available in the foreseeable future.
Other Products
We
have utilized the MUSE
TM
system technological platform for the development of prototypes for other endoscopy and direct
vision products, including a device aiding colonoscopy, a device used in dental surgery and others. To date, we have not yet applied
for regulatory approvals for these devices, nor have we entered into agreements for the commercialization of these devices.
Our
Strategy
Our
primary goal is to generate recurring revenues by driving sales of our MUSE™ system and establishing it as the
standard-of-care procedure and device for the treatment of moderate to severe GERD. We believe that we can achieve this goal by
continuing to accumulate clinical data and promote reimbursement for the procedure in the principal markets of North America,
Europe and Asia. Our strategy includes the following key elements:
Driving
MUSE ™ sales
. We intend to continue to focus on commercializing the MUSE
TM
system in key geographies
and markets. Our distribution network continues to expand for further commercialization beyond Italy and Germany. During 2017,
we entered into additional European distribution agreements in Spain and Switzerland. In Germany and the U.S. we will continue
marketing the MUSE™ through a direct effort at key institutions. In addition, we have successfully completed the
technical testing for CFDA approval in China and have already begun the necessary clinical trial in March 2017.
Collaborating
and co-developing with established companies
. We seek to initiate co-development or licensing collaborations with leading
companies which have existing marketing channels or significant marketing power in critical geographies and sales channels.
Out-licensing
products
. We may consider plans to issue a license for various endoscopic systems which are based on owned and patent-protected
technology which has been developed by us. We continue to work to engage in agreements with companies which produce and market
medical devices, to include the production of systems for the foregoing companies which will be integrated by them in the endoscopic
systems which they produce or that we will develop or produce for them.
Developing
additional products
. Additionally, we intend to develop other products which will be based on the integrated and platform
technology which we have developed to date, including our miniaturized visualization imaging products, combined with our flexible
stapling platform, similar to the MUSE
TM
system. Additional products could include a fully integrated, endoscopic platform
designed for endoscopic surgical tissue dissection or for endoscopic sleeve gastrectomy.
Corporate
Information
Our
registered office and principal place of business are located at Omer Industrial Park, No. 7A, P.O. Box 3030, Omer 8496500, Israel
and our telephone number in Israel is + 972 72 260 2200. Our website address is http://www.medigus.com. The information
contained on our website or available through our website does not constitute part of this prospectus supplement. Our registered
agent in the United States is Medigus USA LLC. The address of Medigus USA LLC is 140 Town & Country Dr., Suite C, Danville,
CA 94526, USA.
THE
OFFERING
|
Securities
offered by us in the offering
|
|
810,000
ADSs representing 40,500,000 ordinary shares.
|
|
|
|
|
|
Total
ordinary shares outstanding immediately after this offering
|
|
191,785,784
ordinary shares.
|
|
|
|
|
|
The
ADSs
|
|
Each
ADS represents 50 ordinary shares. The ADSs will be evidenced by American Depositary
Receipts, or ADRs, executed and delivered by The Bank of New York Mellon, as Depositary.
The
Depositary, as depositary, will be the holder of the ordinary shares underlying your ADSs and you will have rights as
provided in the Deposit Agreement, among us, The Bank of New York Mellon, as Depositary, and all owners and holders from
time to time of ADSs issued thereunder, or the Deposit Agreement, a form of which has been filed as Exhibit 1 to the Registration
Statement on Form F-6 filed by The Bank of New York Mellon with the Securities and Exchange Commission on May 7, 2015.
Subject
to compliance with the relevant requirements set out in the prospectus, you may turn in your ADSs to the Depositary in
exchange for ordinary shares underlying your ADSs.
The
Depositary will charge you fees for such exchanges pursuant to the Deposit Agreement.
You
should carefully read the “Description of our American Depositary Shares” section of the accompanying prospectus
and the Deposit Agreement to better understand the terms of the ADSs.
|
|
|
|
|
|
Offering
Price
|
|
The
offering price is $2.00 per ADS.
|
|
|
|
|
|
Concurrent
private placement
|
|
In
a concurrent private placement, we are selling to the investors who purchased ADSs in this offering warrants to purchase up
to 405,000 ADSs, representing 20,250,000 of our ordinary shares, par value NIS 0.10 per share, at an initial exercise price
of $2.25 per ADS, or the Warrants. The Warrants will be exercisable upon the six-month anniversary of their issuance and will
expire five and a half years following the date of issuance. The Warrants, ADSs issuable upon the exercise of the Warrants
and the ordinary shares represented by such ADSs are being offered pursuant to an exemption from registration provided in
Section 4(a)(2) under the Securities Act of 1933, as amended, or the Securities Act, and Rule 506(b) promulgated thereunder,
and they are not being offered pursuant to this prospectus supplement and the accompanying prospectus. See “Private
Placement of Warrants” on page S-39 of this prospectus supplement for a more complete description of the concurrent
private placement.
|
|
|
|
|
|
Use
of proceeds
|
|
We
currently intend to use the net proceeds from the sale of our ADSs for general corporate purposes, including research and
development related purposes and for potential acquisitions. See “Use of Proceeds” for additional information.
|
|
Listing
|
|
Our
ADSs are listed on NASDAQ under the symbol “MDGS” and our ordinary shares currently trade on the TASE in Israel
under the symbol “MDGS”.
|
|
|
|
|
|
Risk
factors
|
|
Before
deciding to invest in our ADSs, you should carefully consider the risks related to our business, the offering and our securities,
and our location in Israel. See “Risk Factors” on page S-10 of this prospectus supplement and those factors
referred to in “Item 3. Key Information – D. Risk Factors” in our Annual Report on Form 20-F for the year
ended December 31, 2016.
|
|
|
|
|
|
Dividend
Policy
|
|
We
have never declared or paid any cash dividends to our shareholders, and we currently do not expect to declare or pay any cash
dividends in the foreseeable future. See “Dividend Policy” on page S-39 of this prospectus supplement.
|
|
|
|
|
|
Depositary
|
|
The
Bank of New York Mellon.
|
The number of ordinary
shares to be outstanding immediately after the offering as shown above is based on 151,285,784 ordinary shares outstanding as of
November 24, 2017. This number does not include, as of such date (i) 12,667,625 ordinary shares issuable upon the exercise of outstanding
options to purchase 12,667,625 ordinary shares at a weighted average exercise price of NIS 0.58 per share or $0.17 per share (based
on the exchange rate reported by the Bank of Israel on such date), equivalent to 253,353 ADSs at a weighted average exercise price
of $8.26 per ADS, (ii) 120,659,480 ordinary shares issuable upon the exercise of outstanding warrants to purchase 120,659,480 ordinary
shares at a weighted average exercise price of NIS0.41 per share or $0.12 per share (based on the exchange rate reported by the
Bank of Israel on such date), equivalent to 2,413,190 ADSs at a weighted average exercise price of $5.78 per ADS, (iii) 20,250,000
ordinary shares issuable upon the exercise of warrants to purchase 405,000 ADSs to be issued in our concurrent private placement,
at an exercise price of $2.25 per ADS, and (iv) 2,835,000 ordinary shares issuable upon the exercise of warrants to purchase 56,700
ADSs at an exercise price of $2.50 per ADS, to be issued to the placement agent in connection with the offering.
Unless
otherwise stated, outstanding share information throughout this prospectus supplement excludes such outstanding securities.
SUMMARY
FINANCIAL DATA
We
derived the summary financial statement data for the years ended December 31, 2014, 2015 and 2016 set forth below from our
audited financial statements and related notes incorporated by reference in this prospectus supplement and the accompanying prospectus.
We derived the summary financial statement data for the nine months ended September 30, 2016 and 2017 from our unaudited condensed
interim financial statements and related notes incorporated by reference in this prospectus supplement and the accompanying prospectus.
Our results for interim periods are not necessarily indicative of the results that may be expected for the entire year. You should
read the information presented below together with our financial statements, the notes to those statements and the other financial
information incorporated by reference in this prospectus supplement and the accompanying prospectus.
|
|
|
Year Ended December 31,
|
|
|
Nine Months Ended
September 30,
|
|
|
|
|
2014
|
|
|
2015
|
|
|
2016
|
|
|
2016
|
|
|
2017
|
|
|
|
|
|
|
|
(unaudited)
|
|
|
|
|
(U.S. Dollars, in thousands, except per share data)
|
|
|
Consolidated Statements of Loss and Other Comprehensive Loss
|
|
|
|
|
Revenues
|
|
$
|
744
|
|
|
$
|
624
|
|
|
$
|
549
|
|
|
$
|
496
|
|
|
$
|
313
|
|
|
Cost of revenues
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Products and services
|
|
|
351
|
|
|
|
277
|
|
|
|
176
|
|
|
|
154
|
|
|
|
145
|
|
|
Inventory impairment
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
297
|
|
|
Gross profit
|
|
|
393
|
|
|
|
347
|
|
|
|
373
|
|
|
|
342
|
|
|
|
(129
|
)
|
|
Operating Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses
|
|
|
4,025
|
|
|
|
4,384
|
|
|
|
3,655
|
|
|
|
3,021
|
|
|
|
1,744
|
|
|
Selling and marketing expenses
|
|
|
2,341
|
|
|
|
2,680
|
|
|
|
2,125
|
|
|
|
1,845
|
|
|
|
541
|
|
|
Administrative and general expenses
|
|
|
2,280
|
|
|
|
2,842
|
|
|
|
3,684
|
|
|
|
3,016
|
|
|
|
2,429
|
|
|
Other income, net
|
|
|
269
|
|
|
|
3
|
|
|
|
-
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
|
|
8,377
|
|
|
|
9,903
|
|
|
|
9,464
|
|
|
|
7,882
|
|
|
|
4,714
|
|
|
Operating loss
|
|
|
(7,984
|
)
|
|
|
(9,556
|
)
|
|
|
(9,091
|
)
|
|
|
(7,540
|
)
|
|
|
(4,843
|
)
|
|
Profit (loss) from changes in fair value of warrants issued to investors
|
|
|
980
|
|
|
|
106
|
|
|
|
25
|
|
|
|
17
|
|
|
|
2,242
|
|
|
Financing income (expenses), net
|
|
|
650
|
|
|
|
(14
|
)
|
|
|
87
|
|
|
|
118
|
|
|
|
51
|
|
|
Loss before taxes on income
|
|
|
(6,354
|
)
|
|
|
(9,464
|
)
|
|
|
(8,979
|
)
|
|
|
(7,405
|
)
|
|
|
(2,550
|
)
|
|
Taxes on income
|
|
|
(4
|
)
|
|
|
(68
|
)
|
|
|
(28
|
)
|
|
|
(24
|
)
|
|
|
(17
|
)
|
|
Loss for the period
|
|
$
|
(6,358
|
)
|
|
$
|
(9,532
|
)
|
|
$
|
(9,007
|
)
|
|
$
|
(7,429
|
)
|
|
$
|
(2,567
|
)
|
|
Other comprehensive income (loss) for the period, net of tax
|
|
|
(1,573
|
)
|
|
|
(211
|
)
|
|
|
-
|
|
|
|
|
|
|
|
-
|
|
|
Total comprehensive loss for the period
|
|
$
|
(7,931
|
)
|
|
$
|
(9,743
|
)
|
|
$
|
(9,007
|
)
|
|
$
|
(7,429
|
)
|
|
$
|
(2,567
|
)
|
|
Basic loss per share
|
|
$
|
(0.33
|
)
|
|
$
|
(0.34
|
)
|
|
$
|
(0.26
|
)
|
|
$
|
(0.22
|
)
|
|
$
|
(0.02
|
)
|
|
Diluted loss per share
|
|
$
|
(0.33
|
)
|
|
$
|
(0.34
|
)
|
|
$
|
(0.26
|
)
|
|
$
|
(0.22
|
)
|
|
$
|
(0.03
|
)
|
|
Weighted average shares – denominator for basic net loss per share
|
|
|
19,500
|
|
|
|
28,415
|
|
|
|
34,397
|
|
|
|
33,369
|
|
|
|
111,865
|
|
|
Denominator for diluted loss per share
|
|
|
19,500
|
|
|
|
28,415
|
|
|
|
34,397
|
|
|
|
33,369
|
|
|
|
117,220
|
|
RISK
FACTORS
You
should carefully consider the risks described below and in our annual report on Form 20-F for the year ended December 31,
2016, as well as the other information included or incorporated by reference in this prospectus supplement and the accompanying
prospectus, including our financial statements and the related notes, before you decide to buy our securities. The risks and uncertainties
described below and incorporated by reference in this prospectus supplement are not the only risks facing us. We may face additional
risks and uncertainties not currently known to us or that we currently deem to be immaterial. Any of the risks described below
or incorporated by reference in this prospectus supplement, and any such additional risks, could materially adversely affect our
business, financial condition or results of operations. In such case, you may lose all or part of your original investment.
Risks
Related to Our Business
Our
auditors have expressed substantial doubt about our ability to continue as a going concern, which may hinder our ability to obtain
further financing.
Our
audited financial statements for the year ended December 31, 2016, were prepared under the assumption that we would continue
our operations as a going concern. Our independent registered public accounting firm has included a “going concern”
explanatory paragraph in its report on our financial statements for the year ended December 31, 2016, indicating that we
have suffered recurring losses from operations and have a net capital deficiency that raises substantial doubt about our ability
to continue as a going concern. Uncertainty concerning our ability to continue as a going concern may hinder our ability to obtain
future financing. Continued operations and our ability to continue as a going concern are dependent on our ability to obtain additional
funding in the near future and thereafter, and there are no assurances that such funding will be available to us at all or will
be available in sufficient amounts or on reasonable terms. Our financial statements do not include any adjustments that may result
from the outcome of this uncertainty. Without additional funds from private and/or public offerings of debt or equity securities,
sales of assets, sales or out-licenses of intellectual property or technologies, or other transactions, we will exhaust our resources
and will be unable to continue operations. If we cannot continue as a viable entity, our shareholders would likely lose most or
all of their investment in us.
Even
if this offering is successful, we will need additional funding. If we are unable to raise capital, we will be forced to reduce
or eliminate our operations.
As
of September 30, 2017, we held approximately $5.8 million in cash and cash equivalents and short term deposits. Based on our projected
cash flows and our cash balances as of the date of this prospectus supplement, our management is of the opinion that without further
fund raising we will not have sufficient resources to enable us to continue advancing our activities and as a result, there is
substantial doubt about our ability to continue as a going concern. At our current burn rate, and without taking into account
the proceeds from this offering, our current cash balance will be sufficient until approximately August 2018, taking into account
shut down costs. With estimated proceeds from this offering totaling approximately $1.4 million, based upon the public offering
price of $2.00 per ADS, after deducting the placement agent fee and estimated offering expenses payable by us, at our expected
burn rate following this offering, the proceeds from this offering will be sufficient until approximately December 2018.
Even
if this offering is successful, if we are unable to obtain additional sufficient financing, we will be forced to reduce the scope
of, or eliminate our operations. We will also have to reduce marketing, customer service or other resources devoted to our products.
Any of these factors will materially harm our business and results of operations.
Our
management’s plans include the continued commercialization of our products, taking cost reduction steps and securing
sufficient financing through the sale of additional equity securities, debt or capital inflows from strategic partnerships.
There are no assurances however, that we will be successful in obtaining the level of financing needed for our operations.
Even
if we are able to continue to finance our business, the sale of additional equity or debt securities could result in dilution
to our current shareholders and could require us to grant a security interest in our assets. If we raise additional funds through
the issuance of debt securities, these securities may have rights senior to those of our ordinary shares and could contain covenants
that could restrict our operations. In addition, we may require additional capital beyond our currently forecasted amounts to
achieve profitability. Any such required additional capital may not be available on reasonable terms, or at all.
During
the past several months we have been implementing a cost reduction program which may be unsuccessful in its execution, and, even
if successful, may lead to undesirable outcomes.
During
the past several months we have been implementing a cost reduction program that has affected the structure and operation of our
business. Such plan reflects assumptions and analyses based on our experience and perception of historical trends, current market
conditions and expected future developments as well as other factors that we consider appropriate under the circumstances. Whether
our cost reduction program will prove successful depends on a number of factors, including but not limited to (i) our ability
to substantially raise additional funding and to obtain adequate liquidity; (ii) our ability to maintain suppliers’, hospitals’,
medical facilities’ and practitioners’ confidence; (iii) our ability to efficiently reduce our operational expenditures,
while retaining key employees; and (iv) the overall success of our business. In addition, as long as these cost reduction measurements
last, and for a substantial time afterwards, our employees may face considerable distraction and uncertainty and we may experience
increased levels of employee attrition. A loss of key personnel could have a material adverse effect on our ability to meet operational
and financial expectations. The pursuit of additional funding and the application of the cost reduction program has occupied and
will continue to occupy a substantial portion of the time and attention of our management and will impact how our business is
conducted.
We
have a history of operating losses and expect to incur additional losses in the future.
We
have sustained losses in recent years, including an operating net loss of $9.1 million and $4.8 million for the year ended December
31, 2016 and the nine months ended September 30, 2017, respectively. We anticipate that we are likely to continue to incur significant
net losses for at least the next several years as we continue the development of the MUSE™ system and potentially other
products, expand our sales and marketing capabilities in the endoscopy-based products market, continue our commercialization of
our MUSE™ system, expand its adoption and clinical implementation, and continue to develop the corporate infrastructure
required to sell and market our products. Our losses have had, and will continue to have, an adverse effect on our shareholders’
equity and working capital. Any failure to achieve and maintain profitability would continue to have an adverse effect on our
shareholders’ equity and working capital and could result in a decline in our share price or cause us to cease operations.
The
future success of our business depends on our ability to continue to develop and obtain regulatory clearances or approvals for
innovative and commercially successful products in our field, which we may be unable to do in a timely manner, or at all. Our
success and ability to generate revenue or be profitable also depends on our ability to establish our sales and marketing force,
generate product sales and control costs, all of which we may be unable to do.
The
commercial success of the MUSE
TM
system or any future product, if approved, depends upon the degree of market acceptance
by physicians, patients, third-party payors, and others in the medical community.
The
commercial success of the MUSE
TM
system and any future product, if approved, depends in part on the medical community,
patients, and third-party payors accepting our products as medically useful, cost-effective, and safe. Any product that we bring
to the market may or may not gain market acceptance by physicians, patients, third-party payors, and others in the medical community.
In addition, since the MUSE
TM
system is a therapeutic device being used for a quality of life, benign disease, market
penetration may be more difficult. To date, we have experienced slower than expected market penetration. If the MUSE
TM
system or any future product, if approved, does not achieve an adequate level of acceptance, we may not generate significant product
revenue and may not become profitable. The degree of market acceptance of these products, if approved for commercial sale, will
depend on a number of factors, including:
|
|
●
|
the
cost, safety, efficacy, and convenience of the MUSE
TM
system and any future product in relation to alternative
treatments and products;
|
|
|
●
|
the
ability of third parties to enter into relationships with us without violating their existing agreements;
|
|
|
●
|
the
effectiveness of our sales and marketing efforts;
|
|
|
●
|
the
prevalence and severity of any side effects resulting from the procedure;
|
|
|
●
|
the
willingness of the target patient population to try new procedures and of physicians to perform new procedures;
|
|
|
●
|
the
strength of marketing and distribution support for, and timing of market introduction of, competing products;
|
|
|
●
|
publicity
concerning our products or competing products and treatments; and
|
|
|
●
|
sufficient
third-party insurance coverage or reimbursement.
|
Even
if the MUSE
TM
system and any future product, if approved, displays a favorable safety and efficacy profile in clinical
trials, market acceptance of the product will not be known until after it is launched. Our efforts to educate the medical community
and third-party payors on the benefits of the products may require significant resources and may never be successful. Such efforts
to educate the marketplace may require more resources than are required by conventional technologies.
Insufficient
coverage or reimbursement from medical insurers to users of our products could harm our ability to market and commercialize our
current and future products.
Our
ability to successfully commercialize our products, mainly the MUSE
TM
system, depends significantly on the availability
of coverage and reimbursement for endoscopic procedures from third-party insurers, including governmental programs, as well as
private insurance and private health plans. Reimbursement is a significant factor considered by hospitals, medical facilities
and practitioners in determining whether to acquire and utilize new capital equipment or to implement new procedures such as our
technology.
In
January 2016, the American Medical Association’s (AMA) Current Procedural Terminology, or CPT, published a new Category
I CPT Code for transoral esophagogastric fundoplasty procedures, which describes procedures conducted with the MUSE
TM
system. In the U.S., the CPT Editorial Panel assigns specific billing codes for physician services and outpatient hospital procedures,
which are used by providers, who are our customers, to bill for procedures. Once a CPT code is established, the Centers
for Medicare and Medicaid Services, or CMS, in turn establishes payment levels and coverage rules under Medicare, and private
payors establish rates and coverage rules. Notwithstanding the issuance of a CPT to report the MUSE procedure and the establishment
of payment rates for the code, we cannot guarantee that the MUSE
TM
system is or will be covered and, if covered, that
reimbursement will be sufficient, and furthermore, we cannot guarantee that the MUSE
TM
system or any future product
will be approved for coverage or reimbursement by Medicare, Medicaid or any third-party payor. Reimbursement decisions in
the European Union and in other jurisdictions outside of the United States vary by country and region and there can be no assurance
that we will be successful in obtaining adequate reimbursement.
We
depend on the success of a limited portfolio of products for our revenue, which could impair our ability to achieve profitability.
Though
we have plans for the development of additional natural orifice surgical products based on our technology including miniature
cameras, flexible stapling and ultrasound, and although we currently derive most of our revenue from the sale of miniature cameras
and related imaging equipment, we plan to derive most of our future revenue from product sales of our imaging equipment and our
flagship MUSE
™
system and its future applications, as well as recurring sales of associated products
required to use the MUSE ™ system. Our future growth and success is dependent on the successful commercialization
of the MUSE
™ system. If we are unable to achieve increased commercial acceptance of the MUSE
™
system, obtain regulatory clearances or approvals for future products, or experience a decrease in the utilization of our product
line or procedure volume, our revenue would be adversely affected.
We
may encounter manufacturing issues during the assembly process of our flagship product
.
Due
to the characteristics of the technologies on which the main parts of the MUSE
™
system are manufactured,
which include plastic and metal injection, sheet metals, laser welding and rubber vulcanization, using production tools such as
molds, templates and jigs, in the event that parts are found which are inaccurate and/or which have been rendered defective and/or
which have failed preliminary tests, we will be forced to repair the manufacturing tools and re-manufacture and/or re-order the
parts, a process which will delay the production timetable. Furthermore, in the event that certain parts are not suitable, due
to a situation whereby the manufacturing tools have not produced the part in the appropriate manner, it may be necessary to redesign
and re-manufacture the manufacturing tool and to manufacture the parts rapidly and at additional cost.
Furthermore,
if we are unable to satisfy commercial demand for our MUSE
™
system due to our inability to assemble,
test and deliver the system in compliance with applicable regulations, our business and financial results, including our ability
to generate revenue, would be impaired, market acceptance of our products could be materially adversely affected and customers
may instead purchase or use competing products.
We
may encounter failure in the operation of our products, which may adversely harm patients operated by using our products.
Users
of our products may encounter failures in mechanical components, which could result in difficulties in operation, or opening or
releasing the products, leading to the need for surgical procedures to correct the mechanical failure, in which case, a patients’
medical condition may worsen.
Additionally,
in the event that users of our products do not follow the instructions for use or the available product training or instructions
(which appear on the screen during the performance of the procedure) the foregoing may cause injury and in certain cases, could
even cause death. A result of this kind could reduce the rate of progress of, or even prevent, the marketing for the MUSE
TM
product and our other products.
Furthermore,
users of our products may encounter failure in electronic components of our products used in the system software, which could
lead to incorrect interpretation by the users or to failure in the operation of the endoscope, and to injury to the patient’s
critical internal organs.
We
have only limited clinical data to support the value of the MUSE ™ system, as well as our other products, which may
make patients, physicians and hospitals reluctant to accept or purchase our products.
Physicians,
hospitals and patients will only accept or purchase our products if they believe them to be safe and effective, with advantages
over competing products or procedures. To date, we have collected only limited clinical data with which to assess our products’
(mainly the MUSE
TM
system) clinical and economic value. The collection of clinical and economic data and the process
of generating peer review publications in support of our product and procedure is an ongoing focus for us.
If
future publications of clinical studies indicate that medical procedures using the MUSE
TM
system are less safe or less
effective than competing products or procedures, patients may choose not to undergo our procedure, and physicians or hospitals
may choose not to purchase or use our system. Furthermore, unsatisfactory patient outcomes or patient injury could cause negative
publicity for our products, particularly in the early phases of product introduction.
Current
economic conditions could delay or prevent our customers from obtaining budgetary approval to purchase a MUSE
TM
system
or other products, which would adversely affect our business, financial condition and results of operations.
As
a result of the concerns relating to the current economic situation or related to ongoing healthcare reimbursement changes, customers
and distributors may be delayed in obtaining, or may not be able to obtain, budgetary approval or financing for their purchases
or leases of medical equipment including our products. These delays may in some instances lead to our customers or distributors
postponing the shipment and use of previously ordered systems and products, cancelling their orders, or cancelling their agreements
with us. An increase in delays and order cancellations of this nature could adversely affect our products sales and revenues and,
therefore, harm our business and results of operations.
In
addition, negative worldwide economic conditions and market instability may make it increasingly difficult for us, our customers,
our distributors and our suppliers to accurately forecast future product demand trends, which could cause us to order or produce
excess products that can increase our inventory carrying costs and result in obsolete inventory. Alternatively, this forecasting
difficulty could cause a shortage of products, or materials used in our products, that could result in an inability to satisfy
demand for our products and a resulting material loss of potential revenue.
Our
reliance on third-party suppliers for most of the components of our products could harm our ability to meet
demand for our products in a timely and cost effective manner.
Though
we attempt to ensure the availability of more than one supplier for each important component in our products, the number
of suppliers engaged in the provision of miniature video sensors which are suitable for our CMOS technology products is very limited,
and therefore in some cases we engage with a single supplier, which may result in dependency on such supplier. This is the case
regarding sensors for the CMOS type technology that is produced by a single supplier in the United States. As we do not have a
contract in place with this supplier, there is no contractual commitment on the part of such supplier for any set quantity of
such sensors. The loss of our sole supplier in providing us with miniature sensors for our CMOS technology products, and our inability
or delay in finding a suitable replacement supplier, could significantly affect our business, financial condition, results of
operations and reputation.
Modifications
to our current regulator-cleared products or the introduction of new products may require new regulatory clearances or approvals
or require us to recall or cease marketing our current products until clearances or approvals are obtained.
Our
MUSE
TM
system has received marketing clearance from the U.S. Food and Drug Administration, or FDA, based on several
510(k) applications, bears the CE Mark (a mark assigned to a product certifying its fulfillment of the Medical Devices Directive
of the European Union), as required in order to market the system in European Union countries and has obtained the necessary license
to market the product in Canada, Turkey and Israel.
Ongoing
modifications to our products may require new regulatory approvals, as with prior 510k clearances, or require us to recall or
cease marketing the modified products until these clearances or approvals are obtained. Any modification to one of our cleared
devices that would constitute a major change in its intended use, or any change that could significantly affect the safety or
effectiveness of the device would require us to obtain a new 510(k) marketing clearance and may even, in some circumstances, require
the submission of a premarket approval, or PMA, track application if the change raises complex or novel scientific issues or the
product has a new intended use. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k)
submission in the first instance, but the FDA may review any manufacturer’s decision. We may make modifications in the future
to the MUSE
TM
system without seeking additional clearances or approvals if we believe such clearances or approvals
are not necessary. However, it is possible that the FDA could change existing policy and practices regarding the assessment of
whether a new 510(k) clearance is required for changes or modifications to existing devices. Under these changed circumstances,
the FDA may disagree with our past or future decisions not to seek a new 510(k) for changes or modifications to existing devices
and require new clearances or approvals. In that case, we may be required to recall and stop marketing our products as modified,
which could require us to redesign our products, conduct clinical trials to support any modifications, and pay significant regulatory
fines or penalties. In addition, the FDA may not approve or clear our products for the indications that are necessary or desirable
for successful commercialization or could require additional clinical trials to support any modifications.
Significant
changes that could be reasonably expected to affect the safety or effectiveness of one of our devices may require us to obtain
a license amendment or possibly a new license from Health Canada, Turkey, or Israel. In addition, we started the process for receiving
a regulatory clearance in China by the CFDA, which could be significantly affected by such changes. Substantial changes to the
quality system or changes to the CE marked device which could affect compliance with the essential requirements of the device
or its intended use must be reported to the Notified Body (an independent and neutral institution appointed to conduct conformity
assessment). This may result in a decision that an existing certificate is valid, an addendum to the certificate is needed or
a new certificate must be obtained. Any failure to maintain our existing clearances or approvals, or delay or failure in obtaining
required clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner,
which in turn would harm our future growth. Any of these actions would harm our operating results. Further, we may also be required
to seek regulatory clearance in additional countries as we expand our marketing efforts.
Moreover,
clearances and approvals by the applicable regulator are subject to continual review, and the later discovery of previously unknown
problems can result in product labeling restrictions or withdrawal of the product from the market. The loss of previously received
approvals or clearances, or the failure to comply with existing or future regulatory requirements could reduce our sales, profitability
and future growth prospects.
We
are currently required by the FDA to refrain from using certain terms to label and market our products, which could harm our ability
to market and commercialize our current or future products.
The
FDA’s 510(k) clearances include a specification of a product’s indication for use, and also authorize specific labeling
and marketing claims and language in promotional materials for the U.S. market. Failure to conform with the specific cleared labeling
of our products or corporate promotional material would be considered mislabeling or off-label promotion which might lead to:
|
|
●
|
untitled
letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
|
|
|
|
|
●
|
customer
notifications, refunds, detention or seizure of our products;
|
|
|
|
|
|
|
●
|
refusing
or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
|
|
|
|
|
|
|
●
|
withdrawing
510(k) marketing clearances or PMA approvals that have already been granted;
|
|
|
|
|
|
|
●
|
refusing
to provide Certificates for Foreign Government;
|
|
|
|
|
|
|
●
|
refusing
to grant export approval for our products; or
|
|
|
|
|
|
|
●
|
pursuing
criminal prosecution.
|
Any
of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our
customers’ demands. We may also be required to bear other costs or take other actions that may have a negative impact on
our future sales and financial condition.
If
we or our third-party manufacturers or suppliers fail to comply with the FDA’s Quality System Regulation or other regulatory
authorities, our manufacturing operations could be interrupted and our product sales and operating results could suffer
.
We
and some of our third-party manufacturers and suppliers are required to comply with the FDA’s Quality System Regulation,
or QSR, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging,
sterilization, storage and shipping of our products. We and our manufacturers and suppliers are also subject to the regulations
of foreign jurisdictions regarding the manufacturing process if we or our distributors market our products abroad. We monitor
our quality management in order to improve our overall level of compliance. Our facilities are subject to periodic and unannounced
inspection by U.S. and foreign regulatory agencies, including notified bodies, to audit compliance with the QSR and comparable
foreign regulations. If our facilities or those of our third-party manufacturers or suppliers are found to be in violation of
applicable laws and regulations, or if we or our manufacturers or suppliers fail to take satisfactory corrective action in response
to an adverse inspection, the regulatory authority could take enforcement action, including any of the following sanctions:
|
|
●
|
untitled
letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
|
●
|
customer
notifications or repair, replacement, refunds, detention or seizure of our products;
|
|
|
●
|
operating
restrictions or partial suspension or total shutdown of production;
|
|
|
●
|
refusing
or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products;
|
|
|
●
|
withdrawing
510(k) marketing clearances that have already been granted, or PMA approvals that we may receive in the future;
|
|
|
●
|
refusing
to provide Certificates for foreign government;
|
|
|
●
|
refusing
to grant export approval for our products; or
|
|
|
●
|
pursuing
criminal prosecution.
|
Any
of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our
customers’ demands, and could have a material adverse effect on our reputation, business, results of operations and financial
condition. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales
and our ability to generate profits.
We
face possible competition from the pharmaceutical sector, which could harm our ability to market and commercialize our current
and future products.
The
development of more powerful drug treatments to assist in the suppression of GERD or other medical problems which compete with
our products, may reduce the size of our target markets and may reduce the need for the use of our systems and products, either
available now, or which will be developed in the future, thus adversely affecting our ability to market and commercialize our
current and future products. While we are unaware of any current pharmaceutical product that could directly compete with the MUSE
TM
system at
this time, there may be new pharmaceutical entrants in the future.
There
can be no assurance that we will be able to compete successfully against current or future competitors or that competition will
not have a material adverse effect on our future revenues and, consequently, on our business, operating results and financial
condition.
We
face competition from medical device companies that develop and market similar related products and systems, or may launch
products in the future, as well as new techniques and devices for treatments performed by our products.
Several
medical device companies have commercial products which compete with the MUSE
TM
system for the treatment
of GERD using an endoscopic method. While we believe that the MUSE
TM
system has several advantages over competing devices,
such as the requirement of one operator, inclusion of visualization and ultrasound apparatuses, use of standard titanium staples,
and reduced risk of harm to adjacent organs, there can be no assurance that we will be able to compete successfully against current
or future competitors or that competition will not have a material adverse effect on our future revenues and, consequently, on
our business, operating results and financial condition.
Reporting
requirements on payments to physicians in the United States may deter doctors from providing advice to the Company.
The
implementation of the reporting and disclosure obligations of the Physician Payment Sunshine Act, which is part of the Affordable
Care Act of 2010, or the Sunshine Act, could adversely affect our business.
The
Sunshine Act has imposed new reporting and disclosure requirements for drug and device manufacturers with regard to payments or
other transfers of value made to certain practitioners (including physicians, dentists and teaching hospitals), and for such manufacturers
and for group purchasing organizations with regard to certain ownership interests held by physicians in the reporting entity.
On February 1, 2013, Centers for Medicare & Medicaid Services, or CMS, released the final rule to implement the
Sunshine Act. Under this rule, data collection activities began on August 1, 2013, and first disclosure reports were due by March
31, 2014, for the period August 1, 2013, through December 31, 2013. As required under the Sunshine Act, CMS publishes information
from these reports on a publicly available website, including amounts transferred and physician, dentist and teaching hospital
identities.
The
final rule implementing the Sunshine Act is complex, ambiguous, and broad in scope. Accordingly, we are required to collect and
report detailed information regarding certain financial relationships we have with U.S. licensed physicians, dentists (if any)
and teaching hospitals in the United States. It is difficult to predict how the new requirements may impact existing relationships
among manufacturers, distributors, physicians, dentists and teaching hospitals. The Sunshine Act preempts similar state reporting
laws, although we, or our subsidiaries, may be required to continue to report under certain of such state laws. While we expect
to have substantially compliant programs and controls in place to comply with the Sunshine Act requirements, and we have completed
our initial registration with CMS and our 2015 report with respect to Sunshine Act reporting, our continued compliance with
the Sunshine Act imposes continuing additional costs on us.
Medical
device development is costly and involves continual technological change which may render our current or future products obsolete.
Innovation
is rapid and continuous in the medical device industry, and our competitors in the medical device industry make significant investments
in research and development. If new products or technologies emerge that provide the same or superior benefits as our products
at equal or lower cost, they could render our products obsolete or unmarketable. We must anticipate changes in the marketplace
and the direction of technological innovation and customer demands. In addition, we face increasing competition from well-financed
medical device companies to develop new technologies and may face competition should we attempt to acquire new technologies, products
and businesses. As a result, we cannot be certain that our products will be competitive with current or future products and technologies.
We
may be subject to product liability claims, product actions, including product recalls, and other field or regulatory actions
that could be expensive, divert management’s attention and harm our business.
Our
business exposes us to potential liability risks, product actions and other field or regulatory actions that are inherent in the
manufacturing, marketing and sale of medical device products. We may be held liable if our products cause injury or death or is
found otherwise unsuitable or defective during usage. The MUSE
TM
system incorporates mechanical and electrical parts,
complex computer software and other sophisticated components, any of which can contain errors or failures. Complex computer software
is particularly vulnerable to errors and failures, especially when first introduced. In addition, new products or enhancements
to our existing products may contain undetected errors or performance problems that, despite testing, are discovered only after
installation.
If
any of our products are defective, whether due to design or manufacturing defects, improper use of the product, or other reasons,
we may voluntarily or involuntarily undertake an action to remove, repair, or replace the product at our expense. In some circumstances
we will be required to notify regulatory authorities of an action pursuant to a product failure.
The
medical device industry has historically been subject to extensive litigation over product liability claims. We anticipate that
as part of our ordinary course of business we will be subject to product liability claims alleging defects in the design, manufacture
or labeling of our products. A product liability claim, regardless of its merit or eventual outcome, could result in significant
legal defense costs and high punitive damage payments. Although we maintain product liability insurance, the coverage may not
be adequate to cover future claims. Additionally, we may be unable to maintain our existing product liability insurance in the
future at satisfactory rates or adequate amounts.
Broad-based
domestic and international government initiatives to reduce spending, particularly those related to healthcare costs, may reduce
reimbursement rates for endoscopic procedures, which will reduce the cost-effectiveness of our products.
Healthcare
reforms, changes in healthcare policies and changes to third-party coverage and reimbursements, including legislation enacted
reforming the U.S. healthcare system, and any future changes to such legislation, may affect demand for our products and may have
a material adverse effect on our financial condition and results of operations. There can be no assurance that current levels
of reimbursement will not be decreased in the future, or that future legislation, regulation, or reimbursement policies of third-parties
will not adversely affect the demand for our products or our ability to sell products on a profitable basis. The adoption of significant
changes to the healthcare system in the United States, Europe or other jurisdictions in which we may market our products, could
limit the prices we are able to charge for our products or the amounts of reimbursement available for our products, could limit
the acceptance and availability of our products, reduce medical procedure volumes and increase operational and other costs. For
example, U.S. President Donald Trump has recently publicly indicated an intent to lower healthcare costs through various potential
initiatives. In addition, President Trump and other U.S. lawmakers have made statements about potentially repealing or replacing
the Affordable Care Act, although specific legislation for such a repeal or replacement is still in its early stages. While we
are unable to predict what changes may ultimately be enacted, to the extent that future changes affect how our products are paid
for and reimbursed by government and private payers our business could be adversely impacted.
We
cannot predict whether future healthcare initiatives will be implemented at the federal or state level or internationally, or
the effect that any future legislation or regulation will have on us. The expansion of government’s role in any country’s
healthcare industry may result in decreased profits to us, lower reimbursements by third-parties for procedures in which our products
are used, and reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results
of operations.
We
depend on key employees, and if we fail to attract and retain employees with the expertise required for our business and provide
for the succession of senior management, we cannot grow or achieve profitability.
We
are dependent on the continued service and performance of members of our senior management and other key personnel, for example
our Chief Executive Officer, Chris Rowland. We do not maintain key-man life insurance. Our future success will depend
in part on our ability to retain our management and scientific teams, to identify, hire and retain additional qualified personnel
with expertise in research and development and sales and marketing, and to effectively provide for the succession of
senior management. Competition for qualified personnel in the medical device industry is intense. We may be unable to replace
key persons if they leave or to fill new positions requiring key persons with appropriate experience.
The
loss of key employees, the failure of any key employee to perform or our inability to attract and retain skilled employees, as
needed, or an inability to effectively plan for and implement a succession plan for key employees could harm our business.
If
we or our contractors or service providers fail to comply with regulatory laws and regulations, we or they could be subject to
regulatory actions, which could affect our ability to develop, market and sell our products and any other or future products that
we may develop and may harm our reputation.
If
we or our manufacturers or other third-party contractors fail to comply with applicable federal, state or foreign laws or regulations,
we could be subject to regulatory actions, which could affect our ability to develop, market and sell our current products or
any future products which we may develop in the future and could harm our reputation and lead to reduced demand for or non-acceptance
of our proposed products by the market.
If
our employees commit fraud or other misconduct, including noncompliance with regulatory standards and requirements and insider
trading, our business may experience serious adverse consequences.
We
are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply
with FDA regulations, to provide accurate information to the FDA, to comply with manufacturing standards we have established,
to comply with federal and state health-care fraud and abuse laws and regulations, to report financial information or data accurately
or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry
are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices.
These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission,
customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information
obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation.
Our
board of directors adopted a Code of Ethics in March 2016. However, it is not always possible to identify and deter employee misconduct,
and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks
or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance
with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves
or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant
fines or other sanctions.
In
addition, during the course of our operations, our directors, executives and employees may have access to material, nonpublic
information regarding our business, our results of operations or potential transactions we are considering. If a director, executive
or employee was to be investigated, or an action was to be brought against a director, executive or employee for insider trading,
it could have a negative impact on our reputation and the market price of the ADSs. Such a claim, with or without merit, could
also result in substantial expenditures of time and money, and divert attention of our management team from other tasks important
to the success of our business.
If
we fail to withhold our position against the Israeli tax authorities in connection with tax withholding, we may be required to
pay additional taxes.
Following
a tax deduction assessment conducted by the Israeli Tax Authorities, or the ITA, in October 2016, we are deemed to be in debt
of approximately $1.52 million of additional withholding taxes, including penalties and interest. It is our management’s
opinion, based on the assessment of our legal counsel, that the chances of the claims of the ITA being dismissed are more likely
than not. Therefore, no allowance regarding this assessment was recorded in our financial statements. However, if our position
is not accepted in the event this case is litigated, our business can be materially adversely affected.
Risks
Related to Our Intellectual Property
If
we are unable to secure and maintain patent or other intellectual property protection for the intellectual property used in our
products, our ability to compete will be harmed.
Our
commercial success depends, in part, on obtaining and maintaining patent and other intellectual property protection for the technologies
used in our products. The patent positions of medical device companies, including ours, can be highly uncertain and involve complex
and evolving legal and factual questions. Furthermore, we might in the future opt to license intellectual property from other
parties. If we, or the other parties from whom we may license intellectual property, fail to obtain and maintain adequate patent
or other intellectual property protection for intellectual property used in our products, or if any protection is reduced or eliminated,
others could use the intellectual property used in our products, resulting in harm to our competitive business position. In addition,
patent and other intellectual property protection may not provide us with a competitive advantage against competitors that devise
ways of making competitive products without infringing any patents that we own or have rights to.
U.S.
patents and patent applications may be subject to interference proceedings, and U.S. patents may be subject to re-examination
proceedings in the U.S. Patent and Trademark Office. Foreign patents may be subject to opposition or comparable proceedings in
the corresponding foreign patent offices. Any of these proceedings could result in loss of the patent or denial of the patent
application, or loss or reduction in the scope of one or more of the claims of the patent or patent application. Changes in either
patent laws or in interpretations of patent laws may also diminish the value of our intellectual property or narrow the scope
of our protection. Interference, re-examination and opposition proceedings may be costly and time consuming, and we, or the other
parties from whom we might potentially license intellectual property, may be unsuccessful in defending against such proceedings.
Thus, any patents that we own or might license may provide limited or no protection against competitors. In addition, our pending
patent applications and those we may file in the future may have claims narrowed during prosecution or may not result in patents
being issued. Even if any of our pending or future applications are issued, they may not provide us with adequate protection or
any competitive advantages. Our ability to develop additional patentable technology is also uncertain.
Non-payment
or delay in payment of patent fees or annuities, whether intentional or unintentional, may also result in the loss of patents
or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws
under which a patent owner may be compelled to grant licenses to other parties. In addition, many countries limit the enforceability
of patents against other parties, including government agencies or government contractors. In these countries, the patent owner
may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries
do not protect intellectual property rights to the same extent as do the laws of the United States, particularly in the field
of medical products and procedures.
If
we are unable to prevent unauthorized use or disclosure of our proprietary trade secrets and unpatented know-how, our ability
to compete will be harmed.
Proprietary
trade secrets, copyrights, trademarks and unpatented know-how are also very important to our business. We rely on a combination
of trade secrets, copyrights, trademarks, confidentiality agreements and other contractual provisions and technical security measures
to protect certain aspects of our technology, especially where we do not believe that patent protection is appropriate or obtainable.
We require our office holders, employees, consultants and distributers of our products
and most third parties (such
as contractors or clinical collaborators) to execute confidentiality agreements in connection with their relationships with us.
However, these measures may not be adequate to safeguard our proprietary intellectual property and conflicts may, nonetheless,
arise regarding ownership of inventions. Such conflicts may lead to the loss or impairment of our intellectual property or
to expensive litigation to defend our rights against competitors who may be better funded and have superior resources. Our office
holders, employees, consultants and other advisors may unintentionally or willfully disclose our confidential information to competitors.
In addition, confidentiality agreements may be unenforceable or may not provide an adequate remedy in the event of unauthorized
disclosure. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time consuming,
and the outcome is unpredictable. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
Unauthorized parties may also attempt to copy or reverse engineer certain aspects of our products that we consider proprietary.
As a result, other parties may be able to use our proprietary technology or information, and our ability to compete in the market
would be harmed.
We
could become subject to patent and other intellectual property litigation that could be costly, result in the diversion of management’s
attention, require us to pay damages and force us to discontinue selling our products.
Our
industry is characterized by competing intellectual property and a substantial amount of litigation over patent and other intellectual
property rights. Determining whether a product infringes a patent involves complex legal and factual issues, and the outcome of
a patent litigation action is often uncertain. No assurance can be given that patents containing claims covering our products,
parts of our products, technology or methods do not exist, have not been filed or could not be filed or issued. Furthermore, our
competitors or other parties may assert that our products and the methods we employ in the use of our products are covered by
U.S. or foreign patents held by them. In addition, because patent applications can take many years to issue and because publication
schedules for pending applications vary by jurisdiction, there may be applications now pending of which we are unaware and which
may result in issued patents which our current or future products infringe. Also, because the claims of published patent applications
can change between publication and patent grant, there may be published patent applications with claims that we infringe. There
could also be existing patents that one or more of our products or parts may infringe and of which we are unaware. As the number
of competitors in the endoscopic procedure market grows, and as the number of patents issued in this area grows, the possibility
of patent infringement claims against us increases.
Infringement
actions and other intellectual property claims and proceedings brought against or by us, whether with or without merit, may cause
us to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management
from our business and harm our reputation. Some of our competitors may be able to sustain the costs of complex patent or intellectual
property litigation more effectively than we can because they have substantially greater resources.
We
cannot be certain that we will successfully defend against allegations of infringement of patents and intellectual property rights
of others. In the event that we become subject to a patent infringement or other intellectual property lawsuit and if the other
party’s patents or other intellectual property were upheld as valid and enforceable and we were found to infringe the other
party’s patents or violate the terms of a license to which we are a party, we could be required to pay damages. We could
also be prevented from selling our products unless we could obtain a license to use technology or processes covered by such patents
or will be able to redesign the product to avoid infringement. A license may not be available at all or on commercially reasonable
terms or we may not be able to redesign our products to avoid infringement. Modification of our products or development of new
products could require us to conduct clinical trials and to revise our filings with the applicable regulatory bodies, which would
be time consuming and expensive. In these circumstances, we may be unable to sell our products at competitive prices or at all,
our business and operating results could be harmed.
For
example, on October 28, 2016, we settled all litigation and administrative proceedings with EndoChoice, Inc., or EndoChoice, including
those actions pending in the U.S. District Court for the District of Delaware C.A. Nos. 15-505-LPS-CJB and C.A. No. 15-1215-LPS-CJB
and the trademark opposition proceedings in the State of Israel involving Trademark Application Nos. 257172, 260433 and 262423.
Under the terms of the confidential settlement, we were granted a covenant not to sue with respect to EndoChoice FUSE-related
trademarks and EndoChoice was granted a non-exclusive license to our U.S. Patent No. 6,997,871 and related patents. Each party
has agreed to bear its own costs and fees associated with the litigation.
We
may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential
information of third parties or, that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Certain
of our employees and personnel were previously employed at universities, medical institutions, or other biotechnology or pharmaceutical
companies. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information
or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants, or independent
contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary
information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these
claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property
rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs
and be a distraction to management and other employees. Furthermore, universities or medical institutions who employ some of our
key employees and personnel in parallel to their engagement by us may claim that intellectual property developed by such person
is owned by the respective academic or medical institution under the respective institution intellectual property policy or applicable
law.
Risks
Related to Regulatory Compliance
If
we fail to comply with the extensive government regulations relating to our business, we may be subject to fines, injunctions
and other penalties that could harm our business.
Our
medical device products and operations are subject to extensive regulation by the FDA, pursuant to the Federal Food, Drug, and
Cosmetic Act, or FDCA, and various other federal, state and foreign governmental authorities. Government regulations and requirements
specific to medical devices are wide ranging and govern, among other things:
|
|
●
|
design,
development and manufacturing;
|
|
|
|
|
|
|
●
|
testing,
labeling and storage;
|
|
|
●
|
clinical
trials;
|
|
|
|
|
|
|
●
|
product
safety;
|
|
|
●
|
marketing,
sales and distribution;
|
|
|
|
|
|
|
●
|
premarket
clearance or approval;
|
|
|
●
|
record
keeping procedures;
|
|
|
|
|
|
|
●
|
advertising
and promotions; and
|
|
|
●
|
product
recalls and field corrective actions.
|
For
the purpose of receiving FDA clearance through the 510(k) track, the applicant must prove, inter alia, that the device subject
to the application is substantially equivalent to one or more products which have already been approved by the FDA (predicate
device). Additionally, the applicant is required to provide a detailed description of the device, including specifications and
technical information, labeling, instructions for use, and the relevant indications for use of the device which is the subject
of the application.
Clinical
trials are usually not required under the 510(k) track, unless the FDA suspects the device subject to application contains new
technical characteristics requiring clinical results regarding safety and efficacy. Clinical trials whose results are attached
to the application for marketing approval are subject to advance approval by the FDA regarding the protocol of the trial of the
Investigative Device Exemption (IDE) type.
Approval
for marketing of medical devices in the United States can be submitted through a PMA, which is required when the device subject
to approval is not substantially equivalent to a previously approved device, particularly high risk life-saving devices.
Though
the PMA track consists of more stringent requirements than the 510(k) track and can be expensive and lengthy and entail significant
fees, unless exempt. The FDA’s 510(k) marketing clearance process usually takes from three to 12 months, but it can last
longer. The process of obtaining PMA approval is more expensive and uncertain than the 510(k) marketing clearance process. It
generally takes from one to three years, or even longer, from the time the PMA application is submitted to the FDA, until an approval
is obtained. There is no assurance that we will be able to obtain FDA clearance or approval for any new products on a timely basis,
or at all.
In
addition, we are subject to annual regulatory audits in order to maintain our quality system certifications, CE mark permissions,
FDA Clearance and Canadian medical device license. We do not know whether we will be able to continue to affix the CE mark for
new or modified products or that we will continue to meet the quality and safety standards required to maintain the permissions
and license we have already received. If we are unable to maintain our quality system certifications and permission to affix the
CE mark to our products, we will no longer be able to sell our products in member countries of the European Union or other areas
of the world that require CE’s or FDA’s approval of medical devices. If we are unable to maintain our quality system
certifications and Canadian medical device license, we will not be able to sell our products in Canada.
Our
medical device products and operations are also subject to regulation by the Medical Devices and Accessories Division in the Israeli
Ministry of Health, or AMAR, which is responsible for the registration of medical devices in Israel, issuance of import licenses
and monitoring marketing of medical equipment. We have received an AMAR approval in Israel.
Failure
to obtain regulatory approval in additional foreign jurisdictions will prevent us from expanding the commercialization of our
products
.
To
be able to market and sell our products in most other countries, we must obtain regulatory approvals and comply with the regulations
of those countries. These regulations, including the requirements for approvals and the time required for regulatory review, vary
from country to country. Obtaining and maintaining foreign regulatory approvals are expensive and time consuming, and we
cannot be certain that we will receive regulatory approvals in the various countries in which we plan to market our products.
Failure to obtain or maintain regulatory approval in such countries could have an adverse effect on our financial condition and
results of operations.
Our
products may be subject to product actions in the future that could harm our reputation, business operations and financial results.
The
FDA and similar foreign health or governmental authorities have the authority to require an involuntary recall of commercialized
products in the event of material deficiencies or defects in design, or manufacturing or labeling. In the case of the FDA, the
authority to require a recall must be based on an FDA finding. In addition, foreign governmental bodies have the authority to
require a recall of our products in the event of material deficiencies or defects in design or manufacture. Product actions involving
any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and
results of operations.
If
our products, or malfunction of our products, cause or contribute to adverse medical events such as death or a serious injury,
we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that would materially harm our
business.
Our
marketed products are subject to Medical Device Reporting, or MDR, obligations, which require that we report to the FDA any incident
in which our products may have caused or contributed to a death or serious injury, or in which our products malfunctioned and,
if the malfunction were to recur, it could likely cause or contribute to a death or serious injury. The timing of our obligation
to report under the MDR regulations is triggered by the date we become aware of the adverse event as well as the nature of the
event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize
that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is
an adverse event that is unexpected. In addition, all manufacturers placing medical devices in the European Union, Israel and
Canada markets are legally bound to report any serious or potentially serious incidents involving devices they produce or sell
to the relevant authority in whose jurisdiction the incident occurred.
If
we fail to comply with our reporting obligations, the FDA or other agencies in whose jurisdiction the incident occurred, could
take action including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary
penalties, revocation of our device clearances, seizure of our products, or delay in clearance of future products. Any corrective
action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time
and capital, distract management from operating our business, and may materially harm our reputation and financial results.
We
may be subject to fines, penalties or injunctions if we promote the use of our products for unapproved uses, resulting in damage
to our reputation and business.
Our
promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition
of the promotion of a medical device for a use that has not been cleared or approved by FDA. Use of a device outside its cleared
or approved indications is known as “off-label” use. We are not allowed to promote the MUSE
TM
for off label
use. If the FDA determines that we promote an off-label use, it could request that we modify our promotional materials or subject
us to regulatory or enforcement actions, which could have an adverse impact on our reputation and financial results. Similarly,
a CE mark and an AMAR approval is invalidated if any part of the device is modified or used in a manner that is outside of its
intended use.
Regulatory
reforms may adversely affect our ability to sell our products profitably.
From
time to time, legislation is drafted and introduced in the United States, European Union or other countries in which we operate,
that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of medical
devices. In addition, regulations and guidance may often be revised or reinterpreted by the regulatory authorities in ways that
may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted
or interpretations changed, and what the impact of such changes, if any, may be.
On
September 24, 2013, the FDA published a final rule establishing a unique device identification system, or the UDI Rule. The UDI
Rule mandates new labeling requirements that will impact our medical products. We will be required to meet compliance dates as
early as September 24, 2015 for implantable devices (such as staples and cartridges), and additional compliance dates of September
24, 2016 and September 24, 2018 for all other Class II (such as staplers) and reusable components (such as consoles), respectively.
Compliance may involve increases costs and require new equipment, quality systems and manufacturing processes. As of the date
of this prospectus supplement, we are on schedule with the UDI Rule compliance.
If
we fail to comply with federal or state fraud and abuse laws, we could be subject to criminal and civil penalties, loss of licenses
and exclusion from Medicare, Medicaid and other federal and state healthcare programs which could have a material adverse effect
on our business, financial condition and results of operations.
There
are numerous United States federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback laws, false
claims, and physician transparency laws. Section 1128B(b) of the Social Security Act, or the SSA, commonly referred to as the
“Anti-Kickback Statute”, prohibits the offer, payment, solicitation or receipt of any form of remuneration in return
for referring, ordering, leasing, purchasing or arranging for or recommending the ordering, purchasing or leasing of items or
services payable by the Medicare and Medicaid programs or any other federally funded healthcare program. The Anti-Kickback Statute
is very broad in scope, and many of its provisions have not been uniformly or definitively interpreted by courts or regulations.
We have consulting or fee for services arrangements with physicians, hospitals and other entities, which may be subject to scrutiny.
To the extent we are found to not be in compliance, we could face potentially significant fines and penalties in addition to other
more significant sanctions and we may be required to restructure our operations.
Another
development affecting the healthcare industry is the increased use of the federal Civil False Claims Act and, in particular, actions
brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims
Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false
or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private
individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the
federal government, and to share in any monetary recovery. In recent years, the number of suits brought against healthcare providers
by private individuals has increased dramatically. In addition, various states have enacted false claim laws analogous to the
Civil False Claims Act.
The
federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended, created two new federal crimes: healthcare
fraud and false statements relating to healthcare matters. Violations can result in criminal and civil liabilities.
Compliance
with complex foreign and U.S. laws and regulations that apply to our international operations increases our cost of doing business
in international jurisdictions and could expose us or our employees to fines and penalties in the U.S. and abroad. These numerous
and sometimes conflicting laws and regulations include the Foreign Corrupt Practices Act. Many foreign countries have enacted
similar laws addressing fraud and abuse in the healthcare sector. The shifting commercial compliance environment and the need
to build and maintain robust and expandable systems to comply with different compliance requirements in multiple jurisdictions
increases the possibility that a healthcare company may run afoul of one or more of the requirements.
Violations
of any fraud and abuse may result in significant fines, imprisonment and exclusion from the Medicare, Medicaid and other federal
or state healthcare programs which could have a material adverse effect on our business, financial condition and results of operations.
If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us,
we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from federal healthcare
programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. Dealing with investigations can
be time and resource consuming and can divert management’s attention from the business. In addition, settlements with law
enforcement agencies have forced healthcare providers to agree to additional onerous compliance and reporting requirements as
part of a consent decree or corporate integrity agreement. Any violations of these laws, or any action against us for violation
of these laws, even if we successfully defend against it, could have a material adverse effect on our reputation, business and
financial condition. See “Item 4. Information on the Company - B. Business Overview - Fraud and Abuse Laws” in our
Annual Report on Form 20-F for the year ended December 31, 2016.
The
new disclosure rules regarding the use of conflict minerals may affect our relationships with suppliers and customers.
The
Securities and Exchange Commission adopted disclosure rules in August 2012 for companies that use conflict minerals in their products,
with substantial supply chain verification requirements in the event that the materials come from, or could have come from, the
Democratic Republic of the Congo or adjoining countries. These new rules and verification requirements may impose additional costs
on us and on our suppliers, and limit the sources or increase the prices of materials used in our products. Among other things,
this new rule could affect sourcing at competitive prices and availability in sufficient quantities of certain minerals used in
the manufacture of components that are incorporated into our products. In addition, the number of suppliers who provide conflict-free
minerals may be limited, and there may be material costs associated with complying with the disclosure requirements, such as costs
related to the process of determining the source of certain minerals used in our products, as well as costs of possible changes
to products, processes, or sources of supply as a consequence of such verification activities. We may not be able to sufficiently
verify the origins of the relevant minerals used in components manufactured by third parties through the procedures that we implement,
and we may encounter challenges to satisfy those customers who require that all of the components of our products be certified
as conflict-free, which could place us at a competitive disadvantage if we are unable to do so. If we are unable to certify that
our products are conflict free, we may face challenges with our customers, which could place us at a competitive disadvantage,
and our reputation may be harmed.
Risks
Related to Our Operations in Israel
Our
headquarters, manufacturing facilities, and most of our administrative offices are located in Israel and, therefore, our results
may be adversely affected by military instability in Israel.
Our
offices are located in Israel. In addition, the majority of our officers and directors are residents of Israel. Accordingly, geopolitical
or military conditions in Israel and its region may directly or indirectly affect our business. Since the establishment of the
State of Israel in 1948, a number of armed conflicts have taken place between Israel and its neighboring countries. Any hostilities
involving Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our
operations and results of operations. During July and August 2014, Hamas and Israel were engaged in a military conflict that
caused damage and disrupted economic activities in Israel. During November 2012, Hamas and Israel were engaged in an armed
conflict and during the summer of 2006, Israel was engaged in an armed conflict with Hezbollah, a Lebanese Islamist Shiite militia
group and political party. These conflicts involved missile strikes against civilian targets in various parts of Israel, including
areas in which our employees and consultants are located, and negatively affected business conditions in Israel. Any armed conflicts,
terrorist activities or political instability in the region could adversely affect business conditions and could harm our results
of operations and could make it more difficult for us to raise capital. The conflict situation in Israel could cause situations
where medical product certifying or auditing bodies could not be able to visit our manufacturing facilities in order to review
our certifications or clearances, thus possibly leading to temporary suspensions or even cancellations of our clearances or manufacturing
certifications. The conflict situation in Israel could also result in parties with whom we have agreements involving performance
in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions
in such agreements. Furthermore, several countries, principally in the Middle East, restrict doing business with Israel and Israeli
companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities
in the region continue or intensify. Such restrictions may seriously limit our ability to sell our products to customers in those
countries.
Although
the Israeli government is currently committed to covering the reinstatement value of direct damages that are caused by terrorist
attacks or acts of war, we cannot assure that this government coverage will be maintained, or if maintained, will be sufficient
to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our
business. Any armed conflicts would likely negatively affect business conditions generally and could harm our results of operations.
Our
operations may be disrupted as a result of the obligation of management or key personnel to perform military service.
Many
of our male employees in Israel are obligated to perform one month, and in some cases more, of annual military reserve duty until
they reach the age of 40 (or older, for officers or reservists with certain occupations) and, in the event of a military conflict,
may be called to active duty. In response to increases in terrorist activity, there have been periods of significant call-ups
of military reservists, and recently some of our employees have been called up in connection with armed conflicts. It is possible
that there will be military reserve duty call-ups in the future. Our operations could be disrupted by the absence of a significant
number of our employees or of one or more of our key employees. Such disruption could materially adversely affect our business,
financial condition and results of operations.
Exchange
rate fluctuations between the foreign currencies and the NIS may negatively affect our earnings.
Our
reporting and functional currency is the U.S. dollar. Our revenues are currently primarily payable in U.S. dollars and Euros and
we expect our future revenues to be denominated primarily in U.S. dollars and Euros. However, certain amount of our expenses are
in NIS and as a result, we are exposed to the currency fluctuation risks relating to the recording of our expenses in U.S. dollars.
We may, in the future, decide to enter into currency hedging transactions. These measures, however, may not adequately protect
us from material adverse effects
The
government tax benefits that we currently are entitled to receive require us to meet several conditions and may be terminated
or reduced in the future.
Some
of our operations in Israel may entitle us to certain tax benefits under the Law for the Encouragement of Capital Investments,
5719-1959, or the Investments Law, once we begin to produce revenues. From time to time, the government of Israel has considered
reducing or eliminating the tax benefits available to Benefitted Enterprise programs such as ours. If we do not meet the requirements
for maintaining these benefits, they may be reduced or cancelled and the relevant operations would be subject to Israeli corporate
tax at the standard rate, which was set at 24% for 2017 (to be reduced to 23% in 2018 and thereafter). In addition to being subject
to the standard corporate tax rate, we could be required to refund any tax benefits that we have already received, plus interest
and penalties thereon. Even if we continue to meet the relevant requirements, the tax benefits that our current “Benefitted
Enterprise” is entitled to may not be continued in the future at their current levels, or at all. If these tax benefits
were reduced or eliminated, the amount of taxes that we would have to pay if we produce revenues would likely increase, as all
of our operations would consequently be subject to corporate tax at the standard rate, which could adversely affect our results
of operations. Additionally, if we increase our activities outside of Israel, for example, by way of acquisitions, our increased
activities may not be eligible for inclusion in Israeli tax benefits programs. See “Item 10. Additional Information - E. Taxation”
in our Annual Report on Form 20-F for the year ended December 31, 2016.
In
the past, we received Israeli government grants for certain of our research and development activities. The terms of those grants
may require us, in addition to payment of royalties, to satisfy specified conditions in order to manufacture products and transfer
technologies outside of Israel, including increase of the amount of our liabilities in connection with such grants. If we fail
to comply with the requirements of the Innovation Law (as defined below), we may be required to pay penalties in addition to repayment
of the grants, and may impair our ability to sell our technology outside of Israel.
Some
of our research and development efforts were financed in part through royalty-bearing grants, in an amount of $0.2 million
that we received from the Israeli National Authority for Technological Innovation of the Israeli Ministry of Economy and Industry,
or NATI. When know-how is developed using OCS grants, the Encouragement of Research, Development and Technological Innovation
in the Industry Law 5744-1984 (formerly known as the Law for the Encouragement of Research and Development in Industry 5744-1984),
or the Innovation Law and the regulations thereunder, restricts our ability to manufacture products and transfer technology and
know-how, developed as a result of NATI funding, outside of Israel.
Under
the Innovation Law and the regulations thereunder, a recipient of NATI grants is required to return the grants by the payment
of royalties of 3% to 6% on the revenues generated from the sale of products (and related services) developed (in whole or
in part) under NATI program up to the total amount of the grants received from NATI, linked to the U.S. dollar and bearing interest
at an annual rate of LIBOR applicable to U.S. dollar deposits, as published on the first business day of each calendar year.
Transfer
of NATI funded know-how and related intellectual property rights outside of Israel, including by way of license for research and
development purpose requires pre-approval by NATU and imposes certain conditions, including, requirement of payment of a redemption
fee calculated according to the formula provided in the Innovation Law which takes into account, among others, the consideration
for such know-how paid to us in the transaction in which the technology is transferred, research and development expenses, the
amount of NATI support, the time of completion of NATI supported research project and other factors, while the redemption fee
will not exceed 600% of the grants amount plus interest. No assurance can be given that approval to any such transfer, if requested,
will be granted and what will be the amount of the redemption fee payable.
Transfer
of NATI funded know-how and related intellectual property rights to an Israeli company requires a pre-approval by NATI and may
be granted if the recipient undertakes to fulfil all the liabilities to NATI and undertakes to abide by the provisions of Innovation
Law, including the restrictions on the transfer of know-how and the manufacturing rights outside of Israel and the obligation
to pay royalties (note that there will be an obligation to pay royalties to NATI from the income received by us in connection
with such transfer transaction as part of the royalty payment obligation). No assurance can be given that approval to any such
transfer, if requested, will be granted.
In
addition, the products may be manufactured outside Israel by us or by another entity only if prior approval is received from NATI
(such approval is not required for the transfer outside of Israel of less than 10% of the manufacturing capacity in the aggregate,
and in such event only a notice to NATI is required). As a condition for obtaining approval to manufacture outside Israel, we
would be required to pay increased royalties, which usually amount to 1% in addition to the standard royalties rate, and also
the total amount of our liability to NATI will be increased to between 120% and 300% of the grants we received from NATI, depending
on the manufacturing volume that is performed outside Israel (less royalties already paid to NATI). This restriction may impair
our ability to outsource manufacturing rights abroad, however, does not restrict export of our products that incorporate NATI
funded know-how.
A
company also has the option of declaring in its NATI grant application its intention to exercise a portion of the manufacturing
capacity abroad, thus avoiding the need to obtain additional approval. Such declaration may affect the increased royalties cap.
The
restrictions under the Innovation Law (such as with respect to transfer of manufacturing rights abroad or the transfer of NATI
funded know-how and related intellectual property rights abroad) will continue to apply even our liabilities to NATI in full and
will cease to exist only upon payment of the redemption fee described above.
Furthermore,
in the event that we undertake a transaction involving the transfer to a non-Israeli entity of technology developed with NATI
funding pursuant to a merger or similar transaction, the consideration available to our shareholders may be reduced by the amounts
we are required to pay to NATI. Any approval, if given, will generally be subject to additional financial obligations. Failure
to comply with the requirements under the Innovation Law may subject us to mandatory repayment of grants received by us (together
with interest and penalties), as well as expose us to criminal proceedings.
The
Innovation Law was amended as of July 29, 2015, or the 2015 Amendment. On January 1, 2016, pursuant to the 2015 Amendment the
National Authority for Technological Innovation, or NATI, was established and in June 2016, NATI was fully constituted. Pursuant
to the 2015 Amendment, NATI is authorized to change the current restrictions imposed on the recipients of grants under the Innovation
Law with a new set of arrangements in connection with ownership obligations of know-how (including with respect to restrictions
on transfer of know-how and manufacturing activities outside of Israel), as well as royalties obligations associated with approved
programs.
In
May 2017 NATI issued new rules for licensing know how developed with NATI funding outside of Israel (the “Licensing Rules”)
allowing us to enter into licensing arrangements or grant other rights in know-how developed under NATI programs outside of Israel,
subject to the prior consent of NATI and payment of license fees to NATI, calculated in accordance with the Licensing Rules. The
payment of the license fees will not discharge us from the obligations to pay royalties or other payments to NATI.
We
were members of an OCS-related consortium, in which certain of our technologies were developed. We are required to provide licenses
to the other members of the consortium to use such technologies for no consideration, which could reduce our profitability.
Certain
of our miniaturized imaging equipment may be based on technological models developed as part of the Bio Medical Photonic Consortium
in the framework of Magnet program of the OCS. The property rights in and to “new information” (as such term is defined
therein) which has been developed by a member of the Consortium, in the framework of a research and development program conducted
as part of the Consortium, belongs solely to the Consortium member that developed it. The developing member is obligated to provide
the other members in the Consortium a non-sublicensable license to use of the “new information” developed by such
member, without consideration, provided that the other members do not transfer such “new information” to any entity
which is not a member of the Consortium, without the consent of such member.
Provisions
of Israeli law and our articles of association may delay, prevent or otherwise impede a merger with, or an acquisition of, our
company, which could prevent a change of control, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli
corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special
approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant
to such types of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the
date on which a merger proposal is filed by each merging company with the Israel Registrar of Companies and at least 30 days
have passed from the date on which the shareholders of both merging companies have approved the merger. In addition, a majority
of each class of securities of the target company must approve a merger. Moreover, a tender offer for all of a company’s
issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95%
of the issued share capital. Completion of the tender offer also requires approval of a majority of the offerees that do not have
a personal interest in the tender offer, unless, following consummation of the tender offer, the acquirer would hold at least
98% of the Company’s outstanding shares. Furthermore, the shareholders, including those who indicated their acceptance of
the tender offer, may, at any time within six months following the completion of the tender offer, claim that the consideration
for the acquisition of the shares does not reflect their fair market value, and petition an Israeli court to alter the consideration
for the acquisition accordingly, unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer
may not seek such appraisal rights, and the acquirer or the company published all required information with respect to the tender
offer prior to the tender offer’s response date.
Furthermore,
Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence
does not have a tax treaty with Israel exempting such shareholders from Israeli tax.
These
and other similar provisions could delay, prevent or impede an acquisition of us or our merger with another company, even if such
an acquisition or merger would be beneficial to us or to our shareholders.
It
may be difficult to enforce a judgment of a U.S. court against us and our officers and directors and the Israeli experts named
in this prospectus supplement in Israel or the U.S., to assert United States securities laws claims in Israel or to serve process
on our officers and directors and these experts.
We
are incorporated in Israel. Certain of our executive officers and directors reside in Israel and most of our assets and most of
the assets of these persons are located outside of the United States. Therefore, a judgment obtained against us, or any of these
persons in the United States, including one based on the civil liability provisions of the U.S. federal securities laws, may not
be collectible in the United States and may not necessarily be enforced by an Israeli court. It may also be difficult to affect
service of process on these persons in the United States or to assert United States securities law claims in original actions
instituted in Israel.
Even
if an Israeli court agrees to hear such claim, it may determine that Israeli law, and not U.S. law is applicable to the claim.
Under Israeli law, if U.S. law is found to be applicable to such claim, the content of applicable U.S. law must be proven as a
fact by expert witnesses, which can be a time consuming and costly process, and certain matters of procedure would also be governed
by Israeli law. There is little binding case law in Israel that addresses the matters. See “Enforceability of Civil Liabilities”
on page 22 of the accompanying prospectus for additional information on your ability to enforce civil claim against us and our
executive officers and directors.
The
rights and responsibilities of a shareholder will be governed by Israeli law which differs in some material respects from the
rights and responsibilities of shareholders of U.S. companies.
The
rights and responsibilities of the holders of our ordinary shares are governed by our articles of association and by Israeli law.
These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders in typical
U.S.-registered corporations. In particular, a shareholder of an Israeli company has certain duties to act in good faith and fairness
towards the company and other shareholders, and to refrain from abusing its power in the company. There is limited case law available
to assist us in understanding the nature of this duty or the implications of these provisions. These provisions may be interpreted
to impose additional obligations and liabilities on holders of our ordinary shares that are not typically imposed on shareholders
of U.S. corporations.
The
ability of any Israeli company to pay dividends is subject to Israeli law and the amount of cash dividends payable may be subject
to devaluation in the Israeli currency.
The
ability of an Israeli company to pay dividends is governed by Israeli law, which provides that cash dividends may be paid only
out of retained earnings or earnings derived over the two most recent fiscal years, whichever is higher, as determined for statutory
purposes in Israeli currency, provided that there is no reasonable concern that payment of a dividend will prevent a company from
satisfying its existing and foreseeable obligations as they become due. In the event of a devaluation of the Israeli currency
against the U.S. dollar, the amount in U.S. dollars available for payment of cash dividends out of prior years’ earnings
will decrease.
The
termination or reduction of tax and other incentives that the Israeli Government provides to domestic companies may increase the
costs involved in operating a company in Israel.
The
Israeli government currently provides major tax and capital investment incentives to domestic companies, as well as grant and
loan programs relating to research and development and marketing and export activities. In recent years, the Israeli Government
has reduced the benefits available under these programs and the Israeli Governmental authorities have indicated that the government
may in the future further reduce or eliminate the benefits of those programs. We currently take advantage of these programs. There
is no assurance that such benefits and programs would continue to be available in the future to us. If such benefits and programs
were terminated or further reduced, it could have an adverse effect on our business, operating results and financial condition.
We
may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could
result in litigation and adversely affect our business.
A
significant portion of our intellectual property has been developed by our employees in the course of their employment for us.
Under the Israeli Patent Law, 5727-1967, or the Patent Law, inventions conceived by an employee in the course and as a result
of or arising from his or her employment with a company are regarded as “service inventions,” which belong to the
employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Patent
Law also provides that if there is no such agreement between an employer and an employee, the Israeli Compensation and Royalties
Committee, or the Committee, a body constituted under the Patent Law, shall determine whether the employee is entitled to remuneration
for his inventions. Decisions by the Committee (which have been upheld by the Israeli Supreme Court on appeal) have created uncertainty
in this area, as it held that employees may be entitled to remuneration for their service inventions despite having specifically
waived any such rights. However, a recent decision by the Committee held that such right can be waived by the employee. The Committee
further held that an explicit reference to the waived right is not necessary in every circumstance in order for the employee’s
waiver of such right to be valid. Such waiver can be formalized in writing or orally or be implied by the actions of the parties
in accordance with the rules of interpretation of Israeli contract law. We generally enter into assignment-of-invention agreements
with our employees pursuant to which such individuals assign to us all rights to any inventions created in the scope of their
employment or engagement with us. Although our employees have agreed to assign to us service invention rights and have specifically
waived their right to receive any special remuneration for such assignment beyond their regular salary and benefits, we may face
claims demanding remuneration in consideration for assigned inventions.
Risks
Related to an Investment in Our Shares and the ADSs
We
may be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes in 2016 or in any subsequent year.
This may result in adverse U.S. federal income tax consequences for U.S. taxpayers that are holders of our ordinary shares or
the ADSs.
We
will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which either (1) at least 75% of our gross
income is “passive income” or (2) on average at least 50% of our assets by value produce passive income or are
held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends,
interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that
gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including
those raised in a public offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income
and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
We do not believe we were a PFIC for 2016 but there can be no assurance that we were not a PFIC in 2016 and will not be a PFIC
in subsequent years, as our operating results for any such years may cause us to be a PFIC. If we are a PFIC in 2016, or any subsequent
year, and a U.S. shareholder does not make an election to treat us as a “qualified electing fund,” or QEF, or make
a “mark-to-market” election, then “excess distributions” to a U.S. shareholder, and any gain realized
on the sale or other disposition of our securities will be subject to special rules. Under these rules: (1) the excess distribution
or gain would be allocated ratably over the U.S. shareholder’s holding period for the securities; (2) the amount allocated
to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed
as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate
of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would
be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, if the IRS determines
that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S.
shareholder to make a timely QEF or mark-to-market election. U.S. shareholders who hold or have held our securities during a period
when we were or are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject
to exceptions for U.S. shareholders who made a timely QEF or mark-to-market election. A U.S. shareholder can make a QEF election
by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. If applicable, upon
request, we will annually furnish U.S. shareholders with information needed in order to complete IRS Form 8621 (which form would
be required to be filed with the IRS on an annual basis by the U.S. shareholder) and to make and maintain a valid QEF election
for any year in which we or any of our subsidiaries are a PFIC.
The
market prices of our ordinary shares and the ADSs are subject to fluctuation, which could result in substantial losses by our
investors.
The
stock market in general and the market prices of our ordinary shares on the TASE and the ADSs on the NASDAQ, in particular, are
or will be subject to fluctuation, and changes in these prices may be unrelated to our operating performance. We anticipate that
the market prices of our ordinary shares and the ADSs will continue to be subject to wide fluctuations. The market price of our
ordinary shares and the ADSs are, and will be, subject to a number of factors, including:
|
|
●
|
announcements
of technological innovations or new products by us or others;
|
|
|
|
|
|
|
●
|
announcements
by us of significant acquisitions, strategic partnerships, in-licensing, out-licensing, joint ventures or capital commitments;
|
|
|
●
|
expiration
or terminations of licenses, research contracts or other collaboration agreements;
|
|
|
|
|
|
|
●
|
public
concern as to the safety of our equipment we sell;
|
|
|
●
|
general
market conditions;
|
|
|
|
|
|
|
●
|
the
volatility of market prices for shares of medical devices companies generally;
|
|
|
|
|
|
|
●
|
developments
concerning intellectual property rights or regulatory approvals;
|
|
|
●
|
developments
concerning standard-of-care in endoscopic procedures;
|
|
|
|
|
|
|
●
|
variations
in our and our competitors’ results of operations;
|
|
|
●
|
changes
in revenues, gross profits and earnings announced by the company;
|
|
|
|
|
|
|
●
|
changes
in estimates or recommendations by securities analysts, if our ordinary shares or the ADSs are covered by analysts;
|
|
|
●
|
changes
in government regulations or patent decisions; and
|
|
|
|
|
|
|
●
|
general
market conditions and other factors, including factors unrelated to our operating performance.
|
These
factors may materially and adversely affect the market price of our ordinary shares and the ADSs and result in substantial losses
by our investors.
Raising
additional capital by issuing securities may cause dilution to existing shareholders.
We
may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and
strategic and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible
debt securities, the ownership interest will be diluted, and the terms of any such offerings may include liquidation or other
preferences that may adversely affect the then existing shareholders rights. Debt financing, if available, would result in increased
fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific
actions such as incurring debt or making capital expenditures. If we raise additional funds through collaboration, strategic alliance
or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams
or product candidates, or grant licenses on terms that are not favorable to us.
We
do not know whether a market for the ADSs will be sustained or what the trading price of the ADSs will be and as a result it may
be difficult for you to sell your ADSs.
Although
the ADSs now trade on NASDAQ, an active trading market for the ADSs may not be sustained. It may be difficult for you to sell
your ADSs without depressing the market price for the ADSs or at all. As a result of these and other factors, you may not be able
to sell your ADSs. Further, an inactive market may also impair our ability to raise capital by selling ADSs and ordinary Shares
and may impair our ability to enter into strategic partnerships or acquire companies or products by using our ordinary shares
as consideration.
Future
sales of our ordinary shares or the ADSs could reduce the market price of our ordinary shares and the ADSs.
Substantial
sales of our Ordinary Shares or the ADSs, either on the TASE or on NASDAQ, may cause the market price of our ordinary shares or
ADSs to decline. All of our outstanding ordinary shares are registered and available for sale in Israel. Sales by us or our security
holders of substantial amounts of our ordinary shares or ADSs, or the perception that these sales may occur in the future, could
cause a reduction in the market price of our ordinary shares or ADSs.
The
issuance of any additional ordinary shares, any additional ADSs, or any securities that are exercisable for or convertible into
our ordinary shares or ADSs, may have an adverse effect on the market price of our ordinary shares and the ADSs and will have
a dilutive effect on our existing shareholders and holders of ADSs.
Holders
of the ADSs may not receive the same distributions or dividends as those we make to the holders of our ordinary shares, and, in
some limited circumstances, you may not receive dividends or other distributions on our ordinary shares and you may not receive
any value for them, if it is illegal or impractical to make them available to you
.
The
depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on ordinary
shares or other deposited securities underlying the ADSs, after deducting its fees and expenses. You will receive these distributions
in proportion to the number of ordinary shares your ADSs represent. However, the depositary is not responsible if it decides that
it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make
a distribution to a holder of ADSs if it consists of securities that require registration under the Securities Act of 1933, as
amended, or the Securities Act, but that are not properly registered or distributed under an applicable exemption from registration.
In addition, conversion into U.S. dollars from foreign currency that was part of a dividend made in respect of deposited ordinary
shares may require the approval or license of, or a filing with, any government or agency thereof, which may be unobtainable.
In these cases, the depositary may determine not to distribute such property and hold it as “deposited securities”
or may seek to effect a substitute dividend or distribution, including net cash proceeds from the sale of the dividends that the
depositary deems an equitable and practicable substitute. We have no obligation to register under U.S. securities laws any ADSs,
ordinary shares, rights or other securities received through such distributions. We also have no obligation to take any other
action to permit the distribution of ADSs, ordinary shares, rights or anything else to holders of ADSs. In addition, the depositary
may withhold from such dividends or distributions its fees and an amount on account of taxes or other governmental charges to
the extent the depositary believes it is required to make such withholding. This means that you may not receive the same distributions
or dividends as those we make to the holders of our ordinary shares, and, in some limited circumstances, you may not receive any
value for such distributions or dividends if it is illegal or impractical for us to make them available to you. These restrictions
may cause a material decline in the value of the ADSs.
Holders
of ADSs must act through the depositary to exercise their rights as shareholders of our company.
Holders
of our ADSs do not have the same rights of our shareholders and may only exercise the voting rights with respect to the underlying
ordinary shares in accordance with the provisions of the Deposit Agreement. Under Israeli law and our articles of association,
the minimum notice period required to convene a shareholders meeting is no less than 21 or 35 calendar days, depending on the
proposals on the agenda for the shareholders meeting. When a shareholder meeting is convened, holders of ADSs may not receive
sufficient notice of a shareholders’ meeting to permit them to withdraw their ordinary shares to allow them to cast their
vote with respect to any specific matter. In addition, the Depositary and its agents may not be able to send voting instructions
to holders of ADSs or carry out their voting instructions in a timely manner. We will make all reasonable efforts to cause the
Depositary to extend voting rights to holders of the ADSs in a timely manner, but we cannot assure holders that they will receive
the voting materials in time to ensure that they can instruct the Depositary to vote their ordinary shares underlying the ADSs.
Furthermore, the Depositary and its agents will not be responsible for any failure to carry out any instructions to vote, for
the manner in which any vote is cast or for the effect of any such vote. As a result, holders of ADSs may not be able to exercise
their right to vote and they may lack recourse if their ordinary shares underlying the ADSs are not voted as they requested. In
addition, in the capacity as a holder of ADSs, they will not be able to call a shareholders’ meeting.
We
do not intend to pay any cash dividends on our ordinary shares in the foreseeable future and, therefore, any return on your investment
in our ordinary shares or the ADSs must come from increases in the value and trading price of our ordinary shares and the ADSs.
We
have never declared or paid cash dividends on our ordinary shares and do not anticipate that we will pay any cash dividends on
our ordinary shares in the foreseeable future, therefore, any return on your investment in our ordinary shares or the ADSs must
come from increases in the value and trading price of our ordinary shares and the ADSs.
We
intend to retain our earnings to finance the development and expenses of our business. Any future determination relating to our
dividend policy will be at the discretion of our board of directors and will depend on a number of factors, including future earnings,
our financial condition, operating results, contractual restrictions, capital requirements, business prospects, applicable Israeli
law and other factors our board of directors may deem relevant.
We
are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies
may make our common stock less attractive to investors
.
We
are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act,
and may remain an emerging growth company for up to five years. For so long as we remain an emerging growth company, we are permitted
and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not
emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of
Section 404 of the Sarbanes-Oxley Act of 2002 and not being required to comply with any requirement that may be adopted by the
Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report
providing additional information about the audit and the financial statements. We cannot predict whether investors will find our
securities less attractive if we rely on these exemptions. If some investors find our securities less attractive as a result,
there may be a less active trading market for our securities and the price of our securities may be more volatile.
In
addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying
with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards
until those standards would otherwise apply to private companies. We are choosing to “opt out” of this provision and,
as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out
of the extended transition period is irrevocable.
If
securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our
share price and trading volume could decline.
The
trading market for our ordinary shares and the ADSs will depend on the research and reports that securities or industry analysts
publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will
cover us, or provide favorable coverage. If one or more analysts downgrade our stock or change their opinion of our ordinary shares
and the ADSs, the price of our ordinary shares and the ADSs would likely decline. In addition, if one or more analysts cease coverage
of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause
our share price or trading volume to decline.
Our
ordinary shares and the ADSs will be traded on different markets and this may result in price variations.
Our
ordinary shares have been traded on the TASE since February 2006 and our ADSs have been traded on the NASDAQ since May 15, 2015. Trading
in our securities on these markets takes place in different currencies (dollars on the NASDAQ and NIS on the TASE), and at different
times (resulting from different time zones, different trading days and different public holidays in the United States and Israel).
The trading prices of our ordinary shares and the ADSs on these two markets may differ due to these and other factors. Any decrease
in the price of our securities on one of these markets could cause a decrease in the trading price of our securities on the other
market.
We
incur additional increased costs as a result of the listing of the ADSs for trading on the NASDAQ, and our management is required
to devote substantial time to new compliance initiatives and reporting requirements
.
As a public company
in the United States, we incur significant accounting, legal and other expenses as a result of the listing of the ADSs on the NASDAQ. These
include costs associated with corporate governance requirements of the SEC and the Marketplace Rules of the NASDAQ Stock Market,
as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. These
rules and regulations will increase our legal and financial compliance costs, introduced new costs such as investor relations,
stock exchange listing fees and shareholder reporting, and made some activities more time consuming and costly. Any future
changes in the laws and regulations affecting public companies in the United States and Israel, including Section 404 and other
provisions of the Sarbanes-Oxley Act, the rules and regulations adopted by the SEC and the rules of the Nasdaq Stock Market, as
well as compliance with the applicable full Israeli reporting requirements which currently apply to us as a company listed on the
TASE (for so long as they apply to us, pending shareholder approval by special majority of a change to our TASE reporting requirements
to allow us to report to the TASE in the same manner in which we report to the SEC), will result in increased costs to us as we
respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain
types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and
coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also
make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees
or as executive officers.
As
a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of applicable
SEC and NASDAQ requirements, which may result in less protection than is accorded to investors under rules applicable to domestic
issuers
.
As a foreign private
issuer, we are permitted to follow certain home country corporate governance practices instead of those otherwise required under
the rules of the Nasdaq Stock Market for domestic issuers. For instance, we may follow home country practice in Israel with regard
to: distribution of annual and quarterly reports to shareholders, director independence requirements, director nomination
procedures, approval of compensation of officers, approval of related party transactions, shareholder approval requirements, equity
compensation plans and quorum requirements at shareholders’ meetings. In addition, we follow our home country law, instead
of the rules of the Nasdaq Stock Market, which require that we obtain shareholder approval for certain dilutive events, such as
for the establishment or amendment of certain equity based compensation plans, an issuance that will result in a change of control
of the company, certain transactions other than a public offering involving issuances of a 20% or more interest in the company
and certain acquisitions of the stock or assets of another company. Following our home country governance practices as opposed
to the requirements that would otherwise apply to a U.S. company listed on the Nasdaq Stock Market, may provide less protection
than is accorded to investors under the rules of the Nasdaq Stock Market applicable to domestic issuers. For more information,
see “Item 16G. Corporate Governance - Nasdaq Stock Market Listing Rules and Home Country Practices” in our Annual Report
on Form 20-F for the year ended December 31, 2016.
In
addition, as a foreign private issuer, we are exempt from the rules and regulations under the Securities Exchange Act of 1934,
as amended, or the Exchange Act, related to the furnishing and content of proxy statements, and our officers, directors
and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of
the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial
statements with the SEC as frequently or as promptly as domestic companies whose securities are registered under the Exchange
Act.
We
may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
We
are a foreign private issuer, as such term is defined in Rule 405 under the Securities Act, and therefore, we are not required
to comply with all the periodic disclosure and current reporting requirements of the Exchange Act and related rules and regulations.
Under Rule 405, the determination of foreign private issuer status is made annually on the last business day of an issuer’s
most recently completed second fiscal quarter and, accordingly, the next determination will be made with respect to us on June
30, 2018.
In
the future, we would lose our foreign private issuer status if a majority of our shareholders, directors or management are U.S.
citizens or residents and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. Although
we have elected to comply with certain U.S. regulatory provisions, our loss of foreign private issuer status would make such provisions
mandatory. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly
higher. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S.
domestic issuer forms with the U.S. Securities and Exchange Commission, or the SEC, which are more detailed and extensive than
the forms available to a foreign private issuer. For example, the annual report on Form 10-K requires domestic issuers to disclose
executive compensation information on an individual basis with specific disclosure regarding the domestic compensation philosophy,
objectives, annual total compensation (base salary, bonus, equity compensation) and potential payments in connection with change
in control, retirement, death or disability, while the SEC forms applicable to foreign private issuers permit them to disclose
compensation information on an aggregate basis if executive compensation disclosure on an individual basis is not required or
otherwise has not been provided in the issuer’s home jurisdiction. We disclose individual compensation information, but
this disclosure is not as comprehensive as that required of U.S. domestic issuers since we are not required to disclose more detailed
information in Israel. We intend to continue this practice as long as it is permitted under the SEC’s rules and Israel’s
rules do not require more detailed disclosure. We will also have to mandatorily comply with U.S. federal proxy requirements, and
our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions
of Section 16 of the Exchange Act. We may also be required to modify certain of our policies to comply with good governance practices
associated with U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we may lose
our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available
to foreign private issuers.
If
we are unable to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 as they apply to a foreign private
issuer that is listing on a U.S. exchange for the first time, or our internal control over financial reporting is not effective,
the reliability of our financial statements may be questioned and our ordinary share price and the ADSs price may suffer.
Section
404 of the Sarbanes-Oxley Act requires a company subject to the reporting requirements of the U.S. securities laws to do a comprehensive
evaluation of its and its subsidiaries’ internal control over financial reporting. When applicable, to comply with this
statute, we will be required to document and test our internal control procedures; our management will be required to assess and
issue a report concerning our internal control over financial reporting. In addition, our independent registered public accounting
firm may be required to issue an opinion on the effectiveness of our internal control over financial reporting at a later date.
The
continuous process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. Furthermore,
as our business continues to grow both domestically and internationally, our internal controls will become more complex and will
require significantly more resources and attention to ensure our internal controls remain effective overall. During the course
of its testing, our management may identify weaknesses or deficiencies, which may not be remedied in a timely manner. If our management
cannot favorably assess the effectiveness of our internal controls over financial reporting, or if our independent registered
public accounting firm identifies material weaknesses in our internal control, investor confidence in our financial results may
weaken, and the market price of our ordinary shares or the ADSs may suffer.
We
may not satisfy NASDAQ’s requirements for continued listing. If we cannot satisfy these requirements, NASDAQ
could delist our securities.
Our
ADSs are listed on the NASDAQ under the symbol “MDGS”. To continue to be listed on NASDAQ, we are required to satisfy
a number of conditions, including a minimum bid price of at least $1.00 per share, a market value of our publicly held shares
of at least $1 million and shareholders’ equity of at least $2.5 million.
If
we are delisted from NASDAQ, trading in our securities may be conducted, if available, on the OTC Markets or, if available, via
another market. In the event of such delisting, our shareholders would likely find it significantly more difficult
to dispose of, or to obtain accurate quotations as to the value of our securities, and our ability to raise future capital through
the sale of our securities could be severely limited. In addition, if our securities were delisted from NASDAQ, our ADSs could
be considered a “penny stock” under the U.S. federal securities laws. Additional regulatory requirements
apply to trading by broker-dealers of penny stocks that could result in the loss of an effective trading market for our securities.
Moreover, if our securities were delisted from NASDAQ, we will no longer be exempt from certain provision of the Israeli Securities
Law, and therefore will have increased disclosure requirements.
Risks
Related to this Offering
Our
management team will have immediate and broad discretion over the use of the net proceeds from this offering and may not use them
effectively
.
We
currently intend to use the net proceeds of this offering for general corporate purposes, including marketing, production, and
research and development related purposes. See “Use of Proceeds.” However, our management will have broad discretion
in the application of the net proceeds. Our shareholders may not agree with the manner in which our management chooses to allocate
the net proceeds from this offering. The failure by our management to apply these funds effectively could have a material adverse
effect on our business, financial condition and results of operation. Pending their use, we may invest the net proceeds from this
offering in a manner that does not produce income. The decisions made by our management may not result in positive returns on
your investment and you will not have an opportunity to evaluate the economic, financial or other information upon which our management
bases its decisions.
You
will experience immediate dilution in book value of any ADSs you purchase
.
Because
the price per ADS being offered is substantially higher than our net tangible book value per ADS, you will suffer substantial
dilution in the net tangible book value of any ADSs you purchase in this offering. After giving effect to the sale by us of 810,000
ADSs in this offering, based on a public offering price of $2.00 per ADS and after deducting the placement agent’s fees
and offering expenses payable by us, our pro forma net tangible book value of our ADSs would be approximately $5.4 million, or
approximately $1.41 per ADS, as of September 30, 2017. If you purchase ADSs in this offering, you will suffer immediate and
substantial dilution of our pro forma net tangible book value of approximately $0.59 per ADS. See “Dilution” on page
S-35 for a more detailed discussion of the dilution you will incur in connection with this offering.
ADSs
representing a substantial percentage of our outstanding shares may be sold in this offering, which could cause the price of our
ADSs and ordinary shares
to
decline
.
Pursuant
to this offering, we will sell 810,000 ADSs representing 40,500,000 ordinary shares, or approximately 26.77%, of
our outstanding ordinary shares as of November 24, 2017. In addition, the investors in this offering will receive warrants to
purchase up to 405,000 ADSs representing 20,250,000 ordinary shares, and the placement agent will receive unregistered warrants
to purchase up to 56,700 ADSs representing 2,835,000 ordinary shares. This sale and any future sales of a substantial number
of ADSs in the public market, or the perception that such sales may occur, could materially adversely affect the price of our
ADSs and ordinary shares. We cannot predict the effect, if any, that market sales of those ADSs or the availability of those ADSs
for sale will have on the market price of our ADSs and ordinary shares.
USE
OF PROCEEDS
We
estimate that the net proceeds from the sale of 810,000 of our ADSs representing 40,500,000 ordinary shares in this
offering will be approximately $1.4 million after deducting the placement agent’s fees and offering expenses payable by
us.
We
currently intend to use the net proceeds from the sale of our ADSs for general corporate purposes, including research and development
related purposes and for potential acquisitions. However, we have no present binding commitments or agreements to enter into any
acquisitions.
The
amounts and timing of our actual expenditures will depend upon numerous factors, including the progress of our development and
commercialization efforts, the status of and results from our clinical trials, whether or not we enter into strategic collaborations
or partnerships, and our operating costs and expenditures. Accordingly, our management will have significant flexibility in applying
the net proceeds of this offering. In addition, while we have not entered into any binding agreements or commitments relating
to any significant transaction as of the date of this prospectus supplement, we may use a portion of the net proceeds to pursue
acquisitions, joint ventures and other strategic transactions.
DILUTION
If
you invest in our ADSs, your interest will be diluted immediately to the extent of the difference between the public offering
price per ADS and the as adjusted net tangible book value per ADS after this offering.
The
net tangible book value of our ADSs as of September 30, 2017, was approximately $4.36 million, or approximately $1.44 per ADS. Net
tangible book value per ADS represents the amount of our total tangible assets less total liabilities divided by the total number
of our ordinary shares outstanding as of September 30, 2017, and multiplying such amount by 50 (one ADS represents 50 ordinary
shares).
After
giving effect to the sale of our ADSs offered by this prospectus supplement at the offering price of $2.00 per ADS in connection
with this offering and after deducting the placement agent’s fees and offering expenses payable by us, our pro forma net
tangible book value as of September 30, 2017, would have been approximately $5.4 million, or approximately $1.41 per ADS. This
represents an immediate decrease in net tangible book value of approximately $0.03 per ADS to our existing security holders and
an immediate dilution in the pro forma net tangible book value of approximately $0.59 per ADS to purchasers of our ADSs in this
offering, as illustrated by the following table:
|
Public offering price per ADS
|
|
$
|
2.00
|
|
|
|
|
|
|
|
|
Net tangible book value per ADS at September 30, 2017
|
|
$
|
1.44
|
|
|
|
|
|
|
|
|
Decrease in net tangible book value
per ADS attributable to investors purchasing our ADSs in this offering
|
|
$
|
(0.03
|
)
|
|
|
|
|
|
|
|
Pro forma net tangible book value per
ADS as of September 30, 2017, after giving effect to this offering
|
|
$
|
1.41
|
|
|
|
|
|
|
|
|
Dilution per ADS to investors purchasing
our ADSs in this offering
|
|
$
|
0.59
|
|
The preceding table excludes
as of September 30, 2017 (i) 1,135,000 ordinary shares issuable upon the exercise of outstanding options to purchase 1,135,000
ordinary shares at a weighted average exercise price of NIS 5.56 per share or $1.58 per share (based on the exchange rate reported
by the Bank of Israel on such date), equivalent to 22,700 ADSs at a weighted average exercise price of $78.81 per ADS, (ii) 120,659,480
ordinary shares issuable upon the exercise of outstanding warrants to purchase 120,659,480 ordinary shares at a weighted average
exercise price of NIS 0.41 per share or $0.12 per share (based on the exchange rate reported by the Bank of Israel on such date),
equivalent to 2,413,190 ADSs at a weighted average exercise price of $5.77 per ADS, (iii) 20,250,000 ordinary shares issuable
upon the exercise of warrants to purchase 405,000 ADSs to be issued in our concurrent private placement, at an exercise price
of $2.25 per ADS, and (iv) 2,835,000 ordinary shares issuable upon the exercise of warrants to purchase 56,700 ADSs at an exercise
price of $2.50 per ADS, to be issued to the placement agent in connection with the offering.
CAPITALIZATION
The
following table sets forth our total capitalization as of September 30, 2017:
|
|
●
|
on
an actual basis; and
|
|
|
●
|
on
an as adjusted basis to reflect the sale of 810,000 ADSs representing 40,500,000 ordinary shares at the offering price
of $2.00 per ADS, including warrants to purchase 405,000 ADSs representing 20,250,000 ordinary shares sold in a concurrent
private placement, and the receipt by us of net proceeds of approximately $1.4 million, after deducting the placement agent’s
fees and offering expenses payable by us.
|
The
financial data in the following table should be read in conjunction with our unaudited condensed consolidated financial statements
included in the report of foreign private issuer on Form 6-K furnished to the SEC on November 24, 2017, for the period ended September
30, 2017, which has been incorporated by reference in this prospectus supplement and accompanying prospectus.
|
|
|
As
of September 30, 2017
|
|
|
|
|
Actual
|
|
|
As
Adjusted
|
|
|
|
|
(unaudited,
in thousands,
except share data)
|
|
|
Total debt
(1)
|
|
$
|
2,472
|
|
|
$
|
2,806
|
|
|
Shareholders’ equity
|
|
|
|
|
|
|
|
|
|
Ordinary shares, par value NIS
0.10 per share
|
|
|
4,135
|
|
|
|
5,288
|
|
|
Share premium
|
|
|
54,720
|
|
|
|
54,645
|
|
|
Other reserves
|
|
|
348
|
|
|
|
390
|
|
|
Receipts on account
of warrants
|
|
|
1,057
|
|
|
|
1,057
|
|
|
Accumulated deficit
|
|
|
(55,903
|
)
|
|
|
(55,957
|
)
|
|
Total shareholders’
equity
|
|
|
4,357
|
|
|
|
5,423
|
|
|
Total capitalization
and indebtedness
|
|
$
|
6,829
|
|
|
$
|
8,229
|
|
(1)
Includes $825 thousand which are classified as current liabilities.
The preceding table excludes as of September 30, 2017 (i) 1,135,000 ordinary shares issuable upon the
exercise of outstanding options to purchase 1,135,000 ordinary shares at a weighted average exercise price of NIS 5.56 per share
or $1.58 per share (based on the exchange rate reported by the Bank of Israel on such date), equivalent to 22,700 ADSs at a weighted
average exercise price of $78.81 per ADS, (ii) 120,659,480 ordinary shares issuable upon the exercise of outstanding warrants to
purchase 120,659,480 ordinary shares at a weighted average exercise price of NIS 0.41 per share or $0.12 per share (based on the
exchange rate reported by the Bank of Israel on such date), equivalent to 2,413,190 ADSs at a weighted average exercise price of
$5.77 per ADS, (iii) 20,250,000 ordinary shares issuable upon the exercise of warrants to purchase 405,000 ADSs to be issued in
our concurrent private placement, at an exercise price of $2.25 per ADS, and (iv) 2,835,000 ordinary shares issuable upon the exercise
of warrants to purchase 56,700 ADSs at an exercise price of $2.50 per ADS, to be issued to the placement agent in connection with
the offering.
PRICE
RANGE OF OUR ORDINARY SHARES
Our
ordinary shares have been trading on the TASE under the symbol “MDGS” since February 2006. The following table sets
forth, for the periods indicated, the reported high and low sale prices of our ordinary shares on the TASE in NIS and U.S. dollars.
U.S. dollar per ordinary share amounts are calculated using the U.S. dollar representative rate of exchange on the date for which
the high or low market price is applicable, as reported by the Bank of Israel.
|
|
|
NIS
|
|
|
U.S.
$
|
|
|
|
|
Price
per Ordinary Share*
|
|
|
Price
per Ordinary Share*
|
|
|
|
|
High
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
|
Annual:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2017 (through November 23,
2017)
|
|
|
0.70
|
|
|
|
0.14
|
|
|
|
0.19
|
|
|
|
0.04
|
|
|
2016
|
|
|
2.23
|
|
|
|
0.47
|
|
|
|
0.58
|
|
|
|
0.12
|
|
|
2015
|
|
|
5.89
|
|
|
|
0.26
|
|
|
|
0.71
|
|
|
|
1.48
|
|
|
2014
|
|
|
6.73
|
|
|
|
2.50
|
|
|
|
1.93
|
|
|
|
0.64
|
|
|
2013
|
|
|
11.30
|
|
|
|
5.61
|
|
|
|
2.99
|
|
|
|
1.59
|
|
|
2012
|
|
|
13.60
|
|
|
|
5.66
|
|
|
|
3.55
|
|
|
|
1.41
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarterly:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fourth Quarter 2017 (through November
23, 2017)
|
|
|
0.21
|
|
|
|
0.14
|
|
|
|
0.06
|
|
|
|
0.04
|
|
|
Third Quarter 2017
|
|
|
0.21
|
|
|
|
0.14
|
|
|
|
0.06
|
|
|
|
0.04
|
|
|
Second Quarter 2017
|
|
|
0.26
|
|
|
|
0.15
|
|
|
|
0.07
|
|
|
|
0.04
|
|
|
First Quarter 2017
|
|
|
0.70
|
|
|
|
0.22
|
|
|
|
0.19
|
|
|
|
0.06
|
|
|
Fourth Quarter 2016
|
|
|
1.19
|
|
|
|
0.47
|
|
|
|
0.31
|
|
|
|
0.12
|
|
|
Third Quarter 2016
|
|
|
2.23
|
|
|
|
0.65
|
|
|
|
0.58
|
|
|
|
0.17
|
|
|
Second Quarter 2016
|
|
|
1.42
|
|
|
|
0.74
|
|
|
|
0.38
|
|
|
|
0.19
|
|
|
First Quarter 2016
|
|
|
1.87
|
|
|
|
1.26
|
|
|
|
0.48
|
|
|
|
0.32
|
|
|
Fourth Quarter 2015
|
|
|
3.05
|
|
|
|
1.50
|
|
|
|
0.79
|
|
|
|
0.39
|
|
|
Third Quarter 2015
|
|
|
4.43
|
|
|
|
2.72
|
|
|
|
1.17
|
|
|
|
0.70
|
|
|
Second Quarter 2015
|
|
|
5.89
|
|
|
|
3.65
|
|
|
|
1.48
|
|
|
|
0.92
|
|
|
First Quarter 2015
|
|
|
3.90
|
|
|
|
2.59
|
|
|
|
0.98
|
|
|
|
0.67
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Most Recent Six Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
November 2017 (through November 23,
2017)
|
|
|
0.21
|
|
|
|
0.14
|
|
|
|
0.06
|
|
|
|
0.04
|
|
|
October 2017
|
|
|
0.19
|
|
|
|
0.15
|
|
|
|
0.05
|
|
|
|
0.04
|
|
|
September 2017
|
|
|
0.18
|
|
|
|
0.14
|
|
|
|
0.05
|
|
|
|
0.04
|
|
|
August 2017
|
|
|
0.17
|
|
|
|
0.14
|
|
|
|
0.05
|
|
|
|
0.04
|
|
|
July 2017
|
|
|
0.21
|
|
|
|
0.15
|
|
|
|
0.06
|
|
|
|
0.04
|
|
|
June 2017
|
|
|
0.18
|
|
|
|
0.15
|
|
|
|
0.05
|
|
|
|
0.04
|
|
|
May 2017
|
|
|
0.20
|
|
|
|
0.15
|
|
|
|
0.05
|
|
|
|
0.04
|
|
*
price per ordinary share adjusted to reflect the 10:1 reverse share split effected on November 6, 2015.
On
November 23, 2017, the last reported sales price of our ordinary shares on the TASE was NIS 0.152 per share, or $0.04 per share
(based on the exchange rate reported by the Bank of Israel for such date). On November 23, 2017 the exchange rate of
the NIS to the U.S. dollar was $1.00 = NIS 3.512, as reported by the Bank of Israel.
PRICE
RANGE OF OUR ADSs
Our
ADSs commenced trading on the Nasdaq under the symbol “MDGS” on August 5, 2015.
The
following table sets forth, for the periods indicated, the reported high and low sale prices of our ADSs on the Nasdaq in U.S.
dollars.
|
|
|
U.S.
$
|
|
|
|
|
Price
per ADS*
|
|
|
|
|
High
|
|
|
Low
|
|
|
Annual:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2017 (through November 22,
2017)
|
|
|
10.70
|
|
|
|
1.62
|
|
|
2016
|
|
|
32.50
|
|
|
|
5.23
|
|
|
2015 (commencing August 5, 2015)
|
|
|
51.70
|
|
|
|
25.10
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarterly:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fourth Quarter 2017 (through November
22, 2017)
|
|
|
3.18
|
|
|
|
1.62
|
|
|
Third Quarter 2017
|
|
|
2.94
|
|
|
|
1.84
|
|
|
Second Quarter 2017
|
|
|
3.35
|
|
|
|
2.03
|
|
|
First Quarter 2017
|
|
|
10.7
|
|
|
|
2.91
|
|
|
Fourth Quarter 2016
|
|
|
17.8
|
|
|
|
5.23
|
|
|
Third Quarter 2016
|
|
|
32.50
|
|
|
|
7.63
|
|
|
Second Quarter 2016
|
|
|
19.00
|
|
|
|
10.10
|
|
|
First Quarter 2016
|
|
|
26.80
|
|
|
|
15.00
|
|
|
Fourth Quarter 2015
|
|
|
44.80
|
|
|
|
25.10
|
|
|
Third Quarter 2015 (commencing August
5, 2015)
|
|
|
51.70
|
|
|
|
35.20
|
|
|
|
|
|
|
|
|
|
|
|
|
Most Recent Six Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
November 2017 (through November 22,
2017)
|
|
|
3.18
|
|
|
|
1.62
|
|
|
October 2017
|
|
|
2.47
|
|
|
|
2.13
|
|
|
September 2017
|
|
|
2.58
|
|
|
|
1.85
|
|
|
August 2017
|
|
|
2.32
|
|
|
|
1.84
|
|
|
July 2017
|
|
|
2.94
|
|
|
|
2.17
|
|
|
June 2017
|
|
|
2.58
|
|
|
|
2.04
|
|
|
May 2017
|
|
|
2.70
|
|
|
|
2.03
|
|
*
price per ADS adjusted to reflect the 10:1 reverse share split and the change in the ratio of ordinary shares per ADS to 50 deposited
ordinary shares per ADS effected on March 15, 2017.
On
November 22, 2017, the last reported sales price of our ADSs on the Nasdaq was $2.23 per ADS.
DIVIDEND
POLICY
We
have never declared or paid any cash dividends to our shareholders. We currently anticipate that we will retain future earnings
for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for
the foreseeable future.
The
distribution of dividends may also be limited by the Companies Law, which permits the distribution of dividends only out of retained
earnings or earnings derived over the two most recent fiscal years, whichever is higher, provided that there is no reasonable
concern that payment of a dividend will prevent a company from satisfying its existing and foreseeable obligations as they become
due.
PRIVATE
PLACEMENT OF WARRANTS
Concurrently
with the closing of the sale of ordinary shares in this offering, we also expect to issue to the investors in this offering in
a private placement, warrants to purchase an aggregate of 405,000 of our ADSs, equivalent to 20,250,000 of our ordinary shares.
Each
warrant shall be exercisable at any time after six months following the issuance date at an initial exercise price equal to $2.25
per ADS and have a term of exercise equal to five and a half years from the issuance date. The warrants may be exercised for cash
or on a cashless basis if there is no effective registration statement registering the ADSs issuable upon exercise of the warrants.
Subject to limited exceptions, a holder of warrants will not have the right to exercise any portion of its warrants if the holder,
together with its affiliates, would beneficially own in excess of 4.99% of the number of shares of our ordinary shares outstanding
immediately after giving effect to such exercise, subject to increase or decrease up to 9.99% at the election of the holder, provided
that any increase shall not be effective until 61 days following notice of such election.
The
warrants will be issued without registration under the Securities Act, or state securities laws, in reliance on the exemptions
provided by Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder and in reliance on similar exemptions
under applicable state laws. Accordingly, investors may exercise those warrants and sell the underlying shares only pursuant to
an effective registration statement under the Securities Act covering the resale of those shares, an exemption under Rule 144
under the Securities Act, or another applicable exemption under the Securities Act.
PLAN
OF DISTRIBUTION
We
have entered into an engagement agreement, dated as of November 22, 2017, with H.C. Wainwright & Co., LLC, or the placement
agent. Subject to the terms and conditions contained in the engagement agreement, the placement agent has agreed to act as placement
agent in connection with the offer and sale of our ADSs.
The
placement agent may engage selected dealers to assist in the placement of the ADSs. The placement agent is not purchasing
or selling any of the ADSs offered by us under this prospectus supplement and the accompanying prospectus, nor is it required
to arrange the purchase or sale of any specific number or dollar amount of ADSs. The placement agent has agreed to use reasonable
best efforts to arrange for the sale of the ADSs. There is no required minimum number of ADSs that must be sold as
a condition to completion of this offering. The purchase price for the ADSs has been determined based upon arm’s-length
negotiations between the investors and us.
We
have entered into a securities purchase agreement directly with each investor in connection with this offering, and we will only
sell to investors who have entered into the securities purchase agreement. We currently anticipate that the closing
of the sale of the ADSs offered hereby, as well as the concurrent private placement, will be completed on or about November 28,
2017, subject to customary closing conditions.
The
securities purchase agreement we entered with the investors provides that the obligations of the investors of the ADSs are subject
to certain conditions precedent, including, among other things, the absence of any material adverse change in our business and
the receipt of customary legal opinions, letters and certificates.
Upon
closing, we will deliver to each investor delivering funds the number of ADSs purchased by such investor through the facilities
of The Bank of New York Mellon.
The
placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions
received by the placement agent and any profit realized on the resale of the ADSs sold by the placement agent while acting as
principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, the placement
agent would be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation,
Rule 415(a)(4) under the Securities Act and Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may
limit the timing of purchases and sales of ordinary shares by the placement agent acting as principal. Under these rules and regulations,
the placement agent:
|
|
●
|
may
not engage in any stabilization activity in connection with our securities; and
|
|
|
●
|
may
not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than
as permitted under the Exchange Act, until it has completed its participation in the distribution.
|
Commissions
and Expenses
We
have agreed to pay the placement agent an aggregate cash placement agent fee equal to 7% of the gross proceeds received by us
for this offering. This fee will be distributed among the placement agent and any selected-dealers that it has retained to act
on their behalf in connection with this offering.
The
following table shows per ADS and total cash placement agent’s fees we will pay to the placement agent in connection with
the sale of the ADSs pursuant to this prospectus supplement and the accompanying prospectus assuming the purchase of all of the
ADSs offered hereby:
|
Per ADS placement agent
fee
|
|
$
|
0.14
|
|
|
Total
|
|
$
|
113,400
|
|
In
connection with the offering being made by this prospectus supplement and the accompanying prospectus, we will reimburse the placement
agent for certain non-accountable expenses in connection with this offering. The aggregate amount of such reimbursements will
not exceed $45,000.
We
estimate the total offering expenses of this offering that will be payable by us, excluding the placement agent’s fees,
will be approximately $107,000, which includes legal and printing costs, various other fees and the reimbursement of the placement
agent’s expenses.
Placement
Agent Warrants
In
addition, we have agreed to issue to the placement agent warrants to purchase up to 56,700 ADSs, which represents 7% of the aggregate
number of ADSs sold in this offering at an exercise price of $2.50 per ADS (representing 125% of the offering price), exercisable
for five years from the effective date of this offering. The placement agent warrants will otherwise have substantially the same
terms as the warrants being sold to the investors in the concurrent private placement. Pursuant to FINRA Rule 5110(g), the placement
agent warrants and any ordinary shares issued upon exercise of the placement agent warrants shall not be sold, transferred, assigned,
pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result
in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date
of effectiveness or commencement of sales of this offering, except the transfer of any security: (i) by operation of law or by
reason of our reorganization; (ii) to any FINRA member firm participating in the offering and the officers or partners thereof,
if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period;
(iii) if the aggregate amount of our securities held by the placement agent or related persons do not exceed 1% of the securities
being offered; (iv) that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no
participating member manages or otherwise directs investments by the fund and the participating members in the aggregate do not
own more than 10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities remain subject
to the lock-up restriction set forth above for the remainder of the time period.
Right
of First Refusal
We
have also agreed to give the placement agent, subject to the completion of this offering, a right of first refusal, for twelve
months from the commencement of sales in the offering, to act as our sole book-running manager, sole underwriter or sole placement
agent for any further capital raising transactions undertaken by us and a twelve-month tail fee equal to the cash and warrant
compensation in this offering, if any investor who was contacted by the placement agent provides us with further capital during
such twelve-month period following the expiration or termination of our engagement with the placement agent.
Lock-up
Agreements
We
have agreed not to issue, enter into any agreement to issue or announce the issuance or proposed issuance of any ordinary shares,
ADSs or warrants or any other securities convertible into or exchangeable for ordinary shares except for the ordinary shares offered
in this offering for a period of 30 days after the consummation of this offering.
Our
executive officers and directors have agreed, subject to certain exceptions, not to offer, sell, agree to sell, directly or indirectly,
or otherwise dispose of any ordinary shares, ADSs or warrants or any other securities convertible into or exchangeable for ordinary
shares except for the ordinary shares offered in this offering for a period of 90 days after the consummation of this offering.
Indemnification
We
have agreed to indemnify the placement agent and certain other persons against certain liabilities, including liabilities under
the Securities Act and the Exchange Act, and to contribute to payments that the placement agent may be required to make in respect
of such liabilities.
Determination
of offering price
The
offering price of the securities we are offering was negotiated between us and the investors, in consultation with the placement
agent based on the trading of our ADSs prior to the offering, among other things. Other factors considered in determining the
offering price of the ADSs we are offering include the history and prospects of the company, the stage of development of our business,
our business plans for the future and the extent to which they have been implemented, an assessment of our management, general
conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Listing
Our ADSs are listed
on The Nasdaq Capital Market under the trading symbol “MDGS.”
Other
Relationships
From
time to time, the placement agent has provided, and may provide in the future, various advisory, investment and commercial banking
and other services to us in the ordinary course of business, for which they have received and may continue to receive customary
fees and commissions. However, except as disclosed in this prospectus, we have no present arrangements with the placement agent
for any further services.
The
placement agent in this offering provides us with investment banking services.
The
placement agent in this offering served as our exclusive placement agent in a securities offering we consummated in March 2017,
pursuant to which it received compensation, including warrants to purchase our ADSs.
The
placement agent in this offering served as our exclusive placement agent in a securities offering we consummated in December 2016,
pursuant to which it received compensation, including warrants to purchase our ADSs.
Other
The
securities purchase agreement is included as an exhibit to a Report of Foreign Private Issuer on Form 6-K that we filed with the
SEC and that is incorporated by reference into the registration statement of which this prospectus supplement forms a part.
LEGAL
MATTERS
The
validity of the securities offered hereby and certain matters of Israeli law will be passed upon for us by Meiter, Liquornik,
Geva, Leshem, Tal, Ramat Gan, Israel. Certain matters of United States federal securities law relating to this offering will be
passed upon for us by Zysman, Aharoni, Gayer and Sullivan & Worcester, LLP, New York, New York.
EXPERTS
The
financial statements incorporated in this prospectus supplement by reference to the Annual Report on Form 20-F for the year ended
December 31, 2016 have been so incorporated in reliance on the report (which contains an explanatory paragraph relating to the
Company’s ability to continue as a going concern as described in Note 1c to the financial statements) of Kesselman &
Kesselman, Certified Public Accountants (Isr.), a member firm of PricewaterhouseCoopers International Limited, an independent
registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE
YOU CAN FIND MORE INFORMATION
We
are subject to the informational requirements of the United States Securities Exchange Act of 1934, as amended, and in accordance
therewith file annual and special reports with, and furnish other information to, the SEC. You may read and copy the registration
statement and any other documents we have filed at the SEC, including any exhibits and schedules, at the SEC’s public reference
room at 100 F Street N.E., Washington, D.C. 20549. You may call the SEC at 1-800-SEC-0330 for further information on this public
reference room. In addition, the SEC maintains a web site that contains reports and other information regarding issuers that file
electronically with the SEC. You may access the SEC’s website at
http://www.sec.gov
. These SEC filings are also available
to the public on the Israel Securities Authority’s Magna website at
www.magna.isa.gov.il
and from commercial document
retrieval services.
This
prospectus supplement is part of the registration statement on Form F-3 filed with the SEC in connection with this offering and
does not contain all of the information included in the registration statement. Whenever a reference is made in this prospectus
supplement to any of our contracts or other documents, the reference may not be complete and, for a copy of the contract or document,
you should refer to the exhibits that are a part of the registration statement.
INCORPORATION
OF INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” information into this prospectus supplement, which means that we can disclose
important information to you by referring you to other documents which we have filed or will file with the SEC. We are incorporating
by reference in this prospectus supplement the documents listed below and all amendments or supplements we may file to such documents,
as well as any future filings we may make with the SEC on Form 20-F under the United States Securities Exchange Act of 1934, as
amended before the time that all of the securities offered by this prospectus supplement have been sold or de-registered:
|
|
●
|
The
description of our ordinary shares, par value NIS 0.10 per share, and the American
Depositary Shares representing the ordinary shares, contained in our Registration Statement
on Form 20-F filed with the SEC on May 7, 2015;
|
|
|
|
|
|
|
●
|
our
Annual Report on Form 20-F for the fiscal year ended on December 31, 2016, filed with
the SEC on March 31, 2017; and
|
|
|
|
|
|
|
●
|
our
reports on Form 6-K furnished to the SEC on May 9, 2017, May 15, 2017, May 30, 2017 (exhibits
99.2 and 99.3 only), June 28, 2017, July 10, 2017, July 18, 2017, July 19, 2017, July
26, 2017, August 16, 2017, September 11, 2017, September 18, 2017 (exhibits 99.2 and
99.3 only), the Form 6-K furnished on October 17, 2017 (regarding receipt of first purchase
order from Izasa Hospital), October 18, 2017, the Form 6-K furnished on November 24,
2017 (regarding third quarter 2017 financial results, exhibits 99.2 and 99.3 only) and
the Form 6-K furnished on November 24, 2017 (regarding this offering).
|
In
addition, any reports on Form 6-K submitted to the SEC prior to the termination of the offering that we specifically identify
in such forms as being incorporated by reference into the registration statement of which this prospectus supplement forms a part.
Certain
statements in and portions of this prospectus update and replace information in the above listed documents incorporated by reference.
Likewise, statements in or portions of a future document incorporated by reference in this prospectus supplement may update and
replace statements in and portions of this prospectus supplement or the above listed documents.
We
will provide you without charge, upon your written or oral request, a copy of any of the documents incorporated by reference in
this prospectus, other than exhibits to such documents which are not specifically incorporated by reference into such documents.
Please direct your written or telephone requests to Medigus Ltd., Omer Industrial Park, No. 7A, P.O. Box 3030, Omer
8496500, Israel, Attn: Oded Yatzkan, telephone number +972-72-260-2200. You may also obtain information about us by visiting our
website at
www.medigus.com
. Information contained in our website is not part of this prospectus supplement.
PROSPECTUS
$20,000,000
American Depositary Shares
representing Ordinary Shares,
Ordinary Shares,
Warrants to Purchase
American Depositary Shares,
Subscription Rights and/or
Units

MEDIGUS LTD.
We may offer to the
public from time to time in one or more series or issuances American Depositary Shares, or ADSs, ordinary shares, warrants, subscription
rights and/or units consisting of two or more of these classes or series of securities. Each ADS represents five ordinary shares.
We refer to the ADSs, ordinary shares, warrants, subscription rights and units collectively as “securities” in this
prospectus.
Each time we sell securities
pursuant to this prospectus, we will provide a supplement to this prospectus that contains specific information about the offering
and the specific terms of the securities offered. This prospectus may not be used to consummate a sale of securities by us unless
accompanied by the applicable prospectus supplement. You should read this prospectus and the applicable prospectus supplement carefully
before you invest in our securities.
We
may, from time to time, offer to sell the securities, through public or private transactions, directly or through underwriters,
agents or dealers, on or off the Nasdaq Capital Market or Tel Aviv Stock Exchange Ltd., or the TASE, as applicable, at prevailing
market prices or at privately negotiated prices. If any underwriters, agents or dealers are involved in the sale of any of these
securities, the applicable prospectus supplement will set forth the names of the underwriter, agent or dealer and any applicable
fees, commissions or discounts.
Our
ordinary shares are traded on the TASE, and our ADSs are traded on the Nasdaq Capital Market under the symbol “MDGS.”
The last reported sale price for our ADSs on August 23, 2016 as quoted on the Nasdaq Capital Market was $2.10 per ADS, and the
last reported sale price for our ordinary shares on August 23, 2016 as quoted on the TASE was NIS 1.58 per ordinary share, or
$0.42 per ordinary share (based on the exchange rate reported by the Bank of Israel for such date).
On July 28, 2016, the
aggregate market value of our ordinary shares held by non-affiliates was $5,394,041, based on 32,047,034 ordinary shares outstanding
and a per ordinary share price of $0.43 based on the closing sale price of our ordinary shares and the exchange rate reported by
the Bank of Israel on July 28, 2016. We have not offered any securities pursuant to General Instruction I.B.5 on Form F-3 during
the prior 12 calendar month period that ends on and includes the date of this prospectus.
Investing in
these securities involves a high degree of risk. Please carefully consider the risks discussed in this prospectus under
“Risk Factors” beginning on page 2 and the “Risk Factors” in “Item 3: Key Information- Risk
Factors” of our most recent Annual Report on Form 20-F incorporated by reference in this prospectus and in any
applicable prospectus supplement for a discussion of the factors you should consider carefully before deciding to purchase
these securities.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of the securities being offered by this
prospectus, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus
is August 31, 2016
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part
of a registration statement that we filed with the Securities and Exchange Commission, or SEC, utilizing a “shelf”
registration process. Under this process, we may offer and sell our securities under this prospectus.
Under this shelf registration
process, we may sell the securities described in this prospectus in one or more offerings up to a total price to the public of
$20,000,000. The offer and sale of securities under this prospectus may be made from time to time, in one or more offerings, in
any manner described under the section in this prospectus entitled “Plan of Distribution.”
This prospectus provides
you with a general description of the securities we may offer. Each time we sell securities we will provide a prospectus supplement
that will contain specific information about the terms of that offering. The prospectus supplement may also add, update or change
information contained in this prospectus, and may also contain information about any material federal income tax considerations
relating to the securities covered by the prospectus supplement. You should read both this prospectus and any prospectus supplement
together with additional information under the headings “Where You Can Find More Information” and “Incorporation
of Certain Documents by Reference.”
This summary may not
contain all of the information that may be important to you. You should read this entire prospectus, including the financial statements
and related notes and other financial data incorporated by reference in this prospectus, before making an investment decision.
This summary contains forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly
from the results discussed in the forward-looking statements. Factors that might cause or contribute to such differences include
those discussed in “Risk Factors” and “Forward-Looking Statements.”
When used herein, unless
the context requires otherwise, references to the “Company, “we,” “our,” and “us” refer
to Medigus Ltd., an Israeli company, collectively with its wholly-owned subsidiary, Medigus USA LLC, a Delaware corporation.
MEDIGUS LTD.
This summary highlights
information contained in the documents incorporated herein by reference. Before making an investment decision, you should read
the entire prospectus, and our other filings with the SEC, including those filings incorporated herein by reference, carefully,
including the sections entitled “Risk Factors” and “Forward-Looking Statements.”
Overview
We engage in the development,
production and marketing of innovative medical devices, including flexible surgical staplers with direct vision systems for minimally
invasive medical procedures. Our expertise is in the development, production and marketing of innovative endoscopic surgical devices
for the treatment of Gastroesophageal Reflux Disease, or GERD, a common ailment which is predominantly treated by medical therapy
(e.g., proton pump inhibitors) or in more chronic cases, conventional open or laparoscopic surgery. Our FDA-cleared and CE-marked
product, known as the MUSE™ System, enables a trans-orifice procedure, or scarless procedure through a natural
opening in the body, that requires no incision for the treatment of GERD by reconstruction of the esophageal valve where the stomach
and the esophagus meet. We believe this procedure offers a safe, effective and economical alternative to the current
surgical methods of GERD treatment. In addition, this trans-orifice approach has the ability to provide results which
are equivalent to those of standard surgical procedures while reducing pain and trauma, minimizing hospital stays, and delivering
economic value to hospitals and payors.
The key elements
of the MUSE™ system include a single-use, flexible stapler (also called an Endostapler) containing several sophisticated
innovative technologies such as a surgical stapler, miniature camera and ultrasound sensor, as well as a control console, offering
a video image transmitted from the tip of the Endostapler.
In addition to
the MUSE™ system for the treatment of GERD, we are engaged in the development of other minimally invasive endosurgical tools,
as well as miniaturized imaging equipment for use in medical procedures as well as various industrial applications.
Corporate Information
Our registered
office and principal place of business are located at Omer Industrial Park, No. 7A, P.O. Box 3030, Omer 8496500, Israel
and our telephone number in Israel is + 972 72 260 2200. Our website address is http://www.medigus.com. The
information contained on our website or available through our website does not constitute part of this prospectus. Our
registered agent in the United States is Medigus USA LLC. The address of Medigus USA LLC is 140 Town & Country Dr., Suite
C, Danville, CA 94526, USA.
RISK FACTORS
An investment in our
securities involves a high degree of risk. Our business, financial condition or results of operations could be adversely affected
by any of these risks. You should carefully consider the risk factors discussed under the caption “Item 3: Key Information
-
Risk Factors” in our Annual Report on Form 20-F for the year ended December 31, 2015, and in any other filing we
make with the SEC subsequent to the date of this prospectus, each of which are incorporated herein by reference, and in any supplement
to this prospectus, before making your investment decision. The risks and uncertainties we have described are not the only ones
we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our
operations. Past financial performance may not be a reliable indicator of future performance, and historical trends should not
be used to anticipate results or trends in future periods. If any of these risks actually occurs, our business, business prospects,
financial condition or results of operations could be seriously harmed. This could cause the trading price of our securities to
decline, resulting in a loss of all or part of your investment. Please also read carefully the section below entitled “Forward-Looking
Statements.”
FORWARD-LOOKING STATEMENTS
This prospectus, including
the information incorporated by reference into this prospectus, contains, and any prospectus supplement may contain, statements
that are forward-looking statements within the meaning of Private Securities Litigation Reform Act of 1995. We caution you that
any forward-looking statements presented in this prospectus or any prospectus supplement, or which management may make orally
or in writing from time to time, are based on management’s beliefs and assumptions made by, and information currently available
to, management. Such statements may be preceded by the words “intends,” “may,” “will,” “plans,”
“expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,”
“believes,” “hopes,” “potential” or similar words. These statements are not guarantees of
future performance, are based on certain assumptions and our current and best un
derstanding
of the regulatory status and are subject to various known and unknown risks and uncertainties, many of which are beyond our control,
and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by
such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated
with:
|
|
●
|
the
overall global economic environment;
|
|
|
|
|
|
|
●
|
insufficient
coverage or reimbursement from medical insurers;
|
|
|
|
|
|
|
●
|
the
impact of competition and new technologies;
|
|
|
|
|
|
|
●
|
general
market, political, reimbursement and economic conditions in the countries in which we operate;
|
|
|
|
|
|
|
●
|
projected
capital expenditures and liquidity;
|
|
|
|
|
|
|
●
|
changes
in our strategy;
|
|
|
|
|
|
|
●
|
government
regulations and approvals;
|
|
|
|
|
|
|
●
|
changes
in customers’ budgeting priorities;
|
|
|
|
|
|
|
●
|
litigation
and regulatory proceedings; and
|
|
|
|
|
|
|
●
|
those
factors referred to in “Item 3. Key Information – D. Risk Factors,” “Item 4. Information
on the Company,” and “Item 5. Operating and Financial Review and Prospects” in our Annual Report on
Form 20-F for the year ended December 31, 2015.
|
We caution you to carefully
consider these risks and not to place undue reliance on our forward-looking statements. Except as required by law, we assume no
responsibility for updating any forward-looking statements.
CAPITALIZATION
The table below sets
forth our capitalization as of March 31, 2016. The financial data in the following table should be read in conjunction with our
unaudited interim condensed consolidated financial statements and notes thereto incorporated by reference herein.
|
|
|
As of March 31, 2016
|
|
|
|
|
Actual
|
|
|
|
|
(in thousands)
|
|
|
|
|
|
|
|
Total debt
(1)
|
|
$
|
2,203
|
|
|
Shareholders’ equity
|
|
|
|
|
|
Ordinary shares, par value NIS 0.10 per share
|
|
|
870
|
|
|
Share premium
|
|
|
52,080
|
|
|
Other reserves
|
|
|
99
|
|
|
Receipts on account of warrants
|
|
|
1,532
|
|
|
Accumulated deficit
|
|
|
(47,076
|
)
|
|
Total shareholders’ equity
|
|
|
7,505
|
|
|
Total capitalization and indebtedness
|
|
$
|
9,708
|
|
(1)
Includes
$2,101 thousand which are classified as current liabilities.
PRICE RANGE OF OUR
ORDINARY SHARES
Our ordinary shares
have been trading on the TASE under the symbol “MDGS” since February 2006. The following table sets forth, for the
periods indicated, the reported high and low closing sale prices of our ordinary shares on the TASE in NIS and U.S. dollars. U.S.
dollar per ordinary share amounts are calculated using the U.S. dollar representative rate of exchange on the date for which the
high or low market price is applicable, as reported by the Bank of Israel.
|
|
|
NIS
|
|
|
U.S. $
|
|
|
|
|
Price per Ordinary Share*
|
|
|
Price per Ordinary Share*
|
|
|
|
|
High
|
|
|
Low
|
|
|
High
|
|
|
Low
|
|
|
Annual:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015
|
|
|
5.60
|
|
|
|
1.60
|
|
|
|
1.40
|
|
|
|
0.41
|
|
|
2014
|
|
|
6.54
|
|
|
|
2.55
|
|
|
|
1.87
|
|
|
|
0.65
|
|
|
2013
|
|
|
10.89
|
|
|
|
5.65
|
|
|
|
2.92
|
|
|
|
1.62
|
|
|
2012
|
|
|
13.26
|
|
|
|
5.75
|
|
|
|
3.46
|
|
|
|
1.42
|
|
|
2011
|
|
|
14.36
|
|
|
|
6.74
|
|
|
|
4.03
|
|
|
|
1.87
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarterly:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Second Quarter 2016
|
|
|
1.38
|
|
|
|
0.74
|
|
|
|
0.36
|
|
|
|
0.19
|
|
|
First Quarter 2016
|
|
|
1.79
|
|
|
|
1.28
|
|
|
|
0.45
|
|
|
|
0.32
|
|
|
Fourth Quarter 2015
|
|
|
2.91
|
|
|
|
1.60
|
|
|
|
0.75
|
|
|
|
0.41
|
|
|
Third Quarter 2015
|
|
|
4.14
|
|
|
|
2.74
|
|
|
|
1.09
|
|
|
|
0.70
|
|
|
Second Quarter 2015
|
|
|
5.60
|
|
|
|
3.70
|
|
|
|
1.40
|
|
|
|
0.93
|
|
|
First Quarter 2015
|
|
|
3.82
|
|
|
|
2.61
|
|
|
|
0.95
|
|
|
|
0.67
|
|
|
Fourth Quarter 2014
|
|
|
4.37
|
|
|
|
2.55
|
|
|
|
1.19
|
|
|
|
0.65
|
|
|
Third Quarter 2014
|
|
|
5.12
|
|
|
|
4.27
|
|
|
|
1.49
|
|
|
|
1.19
|
|
|
Second Quarter 2014
|
|
|
5.94
|
|
|
|
4.57
|
|
|
|
1.70
|
|
|
|
1.33
|
|
|
First Quarter 2014
|
|
|
6.54
|
|
|
|
5.23
|
|
|
|
1.87
|
|
|
|
1.49
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Most Recent Six Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
August 2016 (through August 23, 2016)
|
|
|
1.58
|
|
|
|
1.14
|
|
|
|
0.42
|
|
|
|
0.30
|
|
|
July 2016
|
|
|
1.63
|
|
|
|
0.66
|
|
|
|
0.42
|
|
|
|
0.16
|
|
|
June 2016
|
|
|
1.17
|
|
|
|
0.74
|
|
|
|
0.30
|
|
|
|
0.19
|
|
|
May 2016
|
|
|
1.31
|
|
|
|
1.03
|
|
|
|
0.34
|
|
|
|
0.26
|
|
|
April 2016
|
|
|
1.38
|
|
|
|
1.28
|
|
|
|
0.36
|
|
|
|
0.33
|
|
|
March 2016
|
|
|
1.43
|
|
|
|
1.30
|
|
|
|
0.36
|
|
|
|
0.34
|
|
|
February 2016
|
|
|
1.55
|
|
|
|
1.28
|
|
|
|
0.39
|
|
|
|
0.32
|
|
*
price per ordinary share adjusted to reflect the 10:1 reverse share split effected on November 6, 2015.
On
August 23, 2016, the last reported sales price of our ordinary shares on the TASE was NIS
1.58
per
share, or $
0.42
per share (based on the exchange rate reported by the Bank of Israel
for such date). On August
23
, 2016 the exchange rate of the NIS to the
U.S. dollar was $1.00 = NIS
3.77
, as reported by the Bank of Israel.
PRICE RANGE OF OUR
ADSs
Our ADSs commenced trading
on the Nasdaq Capital Market under the symbol “MDGS” on August 5, 2015.
The following table
sets forth, for the periods indicated, the reported high and low closing sale prices of our ADSs on the Nasdaq Capital Market in
U.S. dollars.
|
|
|
U.S. $
|
|
|
|
|
Price per ADS*
|
|
|
|
|
High
|
|
|
Low
|
|
|
Annual:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015
(commencing August 5, 2015)
|
|
|
5.00
|
|
|
|
2.68
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarterly:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Second Quarter 2016
|
|
|
1.90
|
|
|
|
1.01
|
|
|
First Quarter 2016
|
|
|
2.68
|
|
|
|
1.69
|
|
|
Fourth Quarter 2015
|
|
|
4.48
|
|
|
|
2.68
|
|
|
Third Quarter 2015 (commencing August 5, 2015)
|
|
|
5.00
|
|
|
|
3.80
|
|
|
|
|
|
|
|
|
|
|
|
|
Most Recent Six Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
August 2016 (through August 23, 2016)
|
|
|
1.58
|
|
|
|
1.14
|
|
|
July 2016
|
|
|
2.13
|
|
|
|
0.76
|
|
|
June 2016
|
|
|
1.64
|
|
|
|
1.01
|
|
|
May 2016
|
|
|
1.84
|
|
|
|
1.38
|
|
|
April 2016
|
|
|
1.90
|
|
|
|
1.84
|
|
|
March 2016
|
|
|
2.05
|
|
|
|
1.69
|
|
|
February 2016
|
|
|
2.54
|
|
|
|
1.72
|
|
*
price per ADS adjusted to reflect the 10:1 reverse share split and the change in the ratio of ordinary shares per ADS to five
deposited ordinary shares per ADS effected on November 6, 2015.
On
August
23, 2016
, the last reported sales price of our ADSs on the Nasdaq Capital Market was $1.58 per ADS.
USE OF PROCEEDS
Unless otherwise indicated
in an accompanying prospectus supplement, the net proceeds from the sale of securities will be used for general corporate purposes,
including research and development related purposes and for potential acquisitions.
DESCRIPTION OF ORDINARY
SHARES
General
The following is a summary
description of our ordinary shares under our articles of association and does not purport to be complete. Our authorized share
capital consists of 150,000,000 ordinary shares. The par value of our ordinary shares is NIS 0.10 per share, and all issued
and outstanding ordinary shares are fully paid and non-assessable. Holders of paid-up ordinary shares are entitled to participate
equally in the payment of dividends and other distributions and, in the event of liquidation, in all distributions after the discharge
of liabilities to creditors.
Transfer of shares
Our fully paid ordinary
shares are issued in registered form and may be freely transferred under our articles of association, unless the transfer is restricted
or prohibited by another instrument, applicable law or the rules of a stock exchange on which the shares are listed for trade.
The ownership or voting of our ordinary shares by non-residents of Israel is not restricted in any way by our articles of association
or the laws of the State of Israel, except for ownership by nationals of certain countries that are, or have been, in a state of
war with Israel.
Liability to further
capital calls
Our board of directors
may make, from time to time, such calls as it may deem fit upon shareholders with respect to any sum unpaid with respect to shares
held by such shareholders which is not payable at a fixed time. Such shareholder shall pay the amount of every call so made upon
him or her.
Election of Directors
Our ordinary shares
do not have cumulative voting rights for the election of directors. As a result, the holders of a majority of the voting power
represented at a shareholders meeting have the power to elect all of our directors, subject to the special approval requirements
for external directors under the Israeli Companies Law, 5759-1999, or the Companies Law, described in our Annual Report on Form
20-F filed with the SEC on March 30, 2016 under “Item 6. Directors, Senior Management and Employees – C. Board Practices
— Corporate governance practices – External directors”.
Under our articles
of association, our board of directors must consist of not less than three but no more than twelve directors, including two external
directors as required by the Companies Law. Pursuant to our articles of association, other than the external directors, for whom
special election requirements apply under the Companies Law, the vote required to appoint a director is a simple majority vote
of holders of our voting shares participating and voting at the relevant meeting. In addition, our articles of association allow
our board of directors to appoint new directors to fill vacancies on the board of directors to serve until the subsequent annual
general meeting of our shareholders, provided, that the number of directors shall not exceed twelve directors. External directors
are elected for an initial term of three years, may be elected for additional terms of three years each under certain circumstances,
and may be removed from office pursuant to the terms of the Companies Law. For further information on the election and removal
of external directors see our Annual Report on Form 20-F filed with the SEC on March 30, 2016 under “Item 6. Directors,
Senior Management and Employees – C. Board Practices — Corporate governance practices – External directors”.
Dividend and liquidation
rights
We may declare a dividend
to be paid to the holders of our ordinary shares in proportion to their respective shareholdings. Under the Companies Law, dividend
distributions are determined by the board of directors and do not require the approval of the shareholders of a company unless
the company’s articles of association provide otherwise. Our articles of association do not require shareholder approval
of a dividend distribution and provide that dividend distributions may be determined by our board of directors.
Pursuant to the
Companies Law, the distribution amount is limited to the greater of retained earnings or earnings generated over the previous two
years, according to our then last reviewed or audited financial statements, provided that the date of the financial statements
is not more than six months prior to the date of the distribution, or we may distribute dividends that do not meet such criteria
only with court approval. In each case, we are only permitted to distribute a dividend if our board of directors and the court,
if applicable, determines that there is no reasonable concern that payment of the dividend will prevent us from satisfying our
existing and foreseeable obligations as they become due.
In the event of
our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our ordinary
shares in proportion to their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant
of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized
in the future.
Exchange controls
There are currently
no Israeli currency control restrictions on remittances of dividends on our ordinary shares, proceeds from the sale of the shares
or interest or other payments to non-residents of Israel, except for shareholders who are subjects of certain countries that are,
or have been, in a state of war with Israel.
Shareholder Meetings
Under the Companies
Law, we are required to hold an annual general meeting of our shareholders once every calendar year that must be held no later
than 15 months after the date of the previous annual general meeting. All general meetings other than the annual meeting of shareholders
are referred to in our articles of association as extraordinary meetings. Our board of directors may call extraordinary meetings
whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Companies Law
provides that our board of directors is required to convene a special meeting upon the written request of (i) any two of our directors
or one-quarter of the members of our board of directors or (ii) one or more shareholders holding, in the aggregate, either (a)
5% or more of our outstanding issued shares and 1% or more of our outstanding voting power or (b) 5% or more of our outstanding
voting power.
Under the Companies
Law, one or more shareholders holding at least 1% of the voting rights at the general meeting may request that the board of directors
include a matter in the agenda of a general meeting to be convened in the future, provided that it is appropriate to discuss such
a matter at the general meeting.
Subject to the provisions
of the Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings
are the shareholders of record on a date to be decided by the board of directors, which may be between four and 40 days prior to
the date of the meeting. Furthermore, the Companies Law requires that resolutions regarding the following matters must be passed
at a general meeting of our shareholders:
|
|
●
|
amendments to our articles of association;
|
|
|
|
|
|
|
●
|
appointment or termination of our auditors;
|
|
|
|
|
|
|
●
|
appointment of external directors;
|
|
|
|
|
|
|
●
|
approval of certain related party transactions;
|
|
|
|
|
|
|
●
|
increases or reductions of our authorized share capital;
|
|
|
|
|
|
|
●
|
mergers; and
|
|
|
|
|
|
|
●
|
the exercise of our board of directors powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management.
|
Under our articles
of association, we are not required to give notice to our registered shareholders pursuant to the Companies Law, unless otherwise
required by law. The Companies Law requires that a notice of any annual general meeting or extraordinary general meeting be provided
to shareholders at least 21 days prior to the meeting and if the agenda of the meeting includes the appointment or removal of
directors, the approval of transactions with office holders or interested or related parties, or an approval of a merger, or as
otherwise required under applicable law, notice must be provided at least 35 days prior to the meeting.
Voting rights
Voting rights
All our ordinary shares
have identical voting and other rights in all respects.
Quorum requirements
Pursuant to our articles
of association, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before
the shareholders at a general meeting. The quorum required for our general meetings of shareholders consists of at least two shareholders,
present in person or by proxy, holding at least ten percent of the voting rights of the Company. A meeting adjourned for lack of
a quorum will be adjourned to the same day of the following week at the same time and place, or to such other day, time or place
if such is stated in the notice of the meeting. At the reconvened meeting, if a quorum is not present within an half an hour, any
number of shareholders present in person or by proxy shall constitute a lawful quorum.
Vote requirements
Our articles of association
provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by the Companies Law
or by our articles of association. Under the Companies Law, each of (i) the approval of an extraordinary transaction with a controlling
shareholder and (ii) the terms of employment or other engagement of the controlling shareholder of the company or such controlling
shareholder’s relative (even if not extraordinary) requires the approval described in our Annual Report on Form 20-F filed
with the SEC on March 30, 2016 under “Item 6. Directors, Senior Management and Employees – C. Board Practices –
Fiduciary duties and approval of specified related party transactions and compensation under Israeli law – Disclosure of
personal interests of a controlling shareholder and approval of transactions.” Certain transactions with respect to remuneration
of our office holders and directors require further approvals described in our Annual Report on Form 20-F filed with the SEC on
March 30, 2016 under “Item 6. Directors, Senior Management and Employees – C. Board Practices – Fiduciary duties
and approval of specified related party transactions and compensation under Israeli law – Compensation of directors and executive
officers.” Another exception to the simple majority vote requirement is a resolution for the voluntary winding up, or an
approval of a scheme of arrangement or reorganization, of the company pursuant to Section 350 of the Companies Law, which requires
the approval of the majority of the shareholders voting their shares, other than abstainees, holding at least 75% of the voting
rights represented at the meeting, in person, by proxy or by voting deed and voting on the resolution.
Access to corporate records
Under the Companies
Law, shareholders are provided access to minutes of our general meetings, our shareholders register and principal shareholders
register, our articles of association, our financial statements and any document that we are required by law to file publicly with
the Israeli Companies Registrar or the Israel Securities Authority. In addition, shareholders may request to be provided with any
document related to an action or transaction requiring shareholder approval under the related party transaction provisions of the
Companies Law. We may deny this request if we believe it has not been made in good faith or if such denial is necessary to protect
our interest or protect a trade secret or patent.
Modification of class
rights
Under the Companies
Law and our articles of association, the rights attached to any class of shares, such as voting, liquidation and dividend rights,
may be amended by adoption of a resolution by the holders of a majority of the shares of that class present at a separate class
meeting, or otherwise in accordance with the rights attached to such class of shares, as set forth in our articles of association.
Acquisitions under Israeli
law
Full tender offer
A person wishing to
acquire shares of an Israeli public company and who would as a result hold over 90% of the target company’s issued and outstanding
share capital is required by the Companies Law to make a tender offer to all of the company’s shareholders for the purchase
of all of the issued and outstanding shares of the company. A person wishing to acquire shares of a public Israeli company and
who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares is required to make
a tender offer to all of the shareholders who hold shares of the relevant class for the purchase of all of the issued and outstanding
shares of that class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital
of the company or of the applicable class, and more than half of the shareholders who do not have a personal interest in the offer
accept the offer, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law.
However, a tender offer will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued and
outstanding share capital of the company or of the applicable class of shares.
Upon a successful completion
of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder accepted the tender
offer or not, may, within six months from the date of acceptance of the tender offer, petition an Israeli court to determine whether
the tender offer was for less than fair value and that the fair value should be paid as determined by the court. However, under
certain conditions, the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be
entitled to petition the Israeli court as described above.
If a tender offer is
not accepted in accordance with the requirements set forth above, the acquirer may not acquire shares of the company that will
increase its holdings to more than 90% of the company’s issued and outstanding share capital or of the applicable class from
shareholders who accepted the tender offer.
Special tender offer
The Companies Law provides
that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as a result of the
acquisition the purchaser would become a holder of 25% or more of the voting rights in the company. This requirement does not apply
if there is already another holder of at least 25% of the voting rights in the company. Similarly, the Companies Law provides that
an acquisition of shares in a public company must be made by means of a special tender offer if as a result of the acquisition
the purchaser would become a holder of more than 45% of the voting rights in the company, if there is no other shareholder of the
company who holds more than 45% of the voting rights in the company, subject to certain exceptions.
A special tender offer
must be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5%
of the voting power attached to the company’s outstanding shares, regardless of how many shares are tendered by shareholders.
A special tender offer may be consummated only if (i) at least 5% of the voting power attached to the company’s outstanding
shares will be acquired by the offeror and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders
objected to the offer (excluding the purchaser and its controlling shareholder, holders of 25% or more of the voting rights in
the company or any person having a personal interest in the acceptance of the tender offer or any other person acting on their
behalf, including relatives and entities under such person’s control). If a special tender offer is accepted, then the purchaser
or any person or entity controlling it or under common control with the purchaser or such controlling person or entity may not
make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target
company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect
such an offer or merger in the initial special tender offer.
Merger
The Companies Law permits
merger transactions if approved by each party’s board of directors and, unless certain requirements described under the Companies
Law are met, by a majority vote of each party’s shares, and, in the case of the target company, a majority vote of each class
of its shares voted on the proposed merger at a shareholders meeting.
For purposes
of the shareholder vote, unless a court rules otherwise, the merger will not be deemed approved if a majority of the votes of
the shares represented at the shareholders meeting that are held by parties other than the other party to the merger, or by any
person (or group of persons acting in concert) who holds (or hold, as the case may be) 25% or more of the voting rights or the
right to appoint 25% or more of the directors of the other party, vote against the merger. If, however, the merger involves a
merger with a company’s own controlling shareholder or if the controlling shareholder has a personal interest in the merger,
then the merger is instead subject to the same special majority approval that governs all extraordinary transactions with controlling
shareholders (as described in our Annual Report on Form 20-F filed with the SEC on March 30, 2016 under “Item 6. Directors,
Senior Management and Employees – C. Board Practices – Fiduciary duties and approval of specified related party transactions
and compensation under Israeli law – Disclosure of personal interests of a controlling shareholder and approval of transactions”).
If
the transaction would have been approved by the shareholders of a merging company but for the separate approval of each class
or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request
of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking
into account the value to the parties to the merger and the consideration offered to the shareholders of the company.
Upon the request of
a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists
a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging
entities, and may further give instructions to secure the rights of creditors.
In addition, a merger
may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed
by each party with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was approved
by the shareholders of each party.
Borrowing powers
Pursuant to the
Companies Law and our articles of association, our board of directors may exercise all powers and take all actions that are not
required under law or under our articles of association to be exercised or taken by a certain organ of the Company, including the
power to borrow money for company purposes.
Changes in capital
Our articles of association
enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Companies Law and must
be approved by a resolution duly adopted by our shareholders at a general meeting. In addition, transactions that have the effect
of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits,
require the approval of both our board of directors and an Israeli court.
DESCRIPTION OF AMERICAN
DEPOSITARY SHARES
General
The following is a summary
description of our ADSs and does not purport to be complete. Each of our ADSs represents five ordinary shares (or a right to receive
five ordinary shares) deposited with the principal Tel Aviv office of either of Bank Hapoalim or Bank Leumi, as custodian for the
Bank of New York Mellon as the depositary. Each ADS also represents any other securities, cash or other property which
may be held by the depositary. The depositary’s office at which the ADSs will be administered is located at 101
Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at One Wall
Street, New York, New York 10286.
You may hold ADSs
either (A) directly (i) by having an American Depositary Receipt, or ADR, which is a certificate evidencing a specific number of
ADSs, registered in your name, or (ii) by having uncertificated ADSs registered in your name, or (B) indirectly by holding a security
entitlement in ADSs through your broker or other financial institution. If you hold ADSs directly, you are a registered
ADS holder, also referred to as an ADS holder. This description assumes you are an ADS holder. If you hold the ADSs
indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described
in this section. You should consult with your broker or financial institution to find out what those procedures are.
Registered holders of
uncertificated ADSs will receive statements from the depositary confirming their holdings. As an ADS holder, we will not treat
you as one of our shareholders and you will not have shareholder rights. Israeli law governs shareholder rights. The
depositary will be the holder of the shares underlying your ADSs. As a registered holder of ADSs, you will have ADS
holder rights. A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially
holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs
the deposit agreement and the ADSs.
The following
is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire deposit
agreement and the form of ADR.
Dividends and Other
Distributions
How will you receive
dividends and other distributions on the shares?
The depositary has agreed
to pay to ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities,
after deducting its fees and expenses. You will receive these distributions in proportion to the number of shares your
ADSs represent.
Cash
. The depositary
will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on a reasonable
basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government approval is needed and
cannot be obtained, the deposit agreement allows the depositary to distribute the NIS only to those ADS holders to whom it is possible
to do so. It will hold the NIS it cannot convert for the account of the ADS holders who have not been paid. It will not invest
the NIS and it will not be liable for any interest.
Before making a distribution,
any withholding taxes, or other governmental charges that must be paid will be deducted. For more information see “Item 10.
Additional Information – E. Taxation” in our Annual Report on Form 20-F filed with the SEC on March 30,
2016. The depositary will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole
cent.
If the exchange rates fluctuate during a time when the depositary cannot convert the NIS, you may lose some
or all of the value of the distribution.
Shares.
The
depositary may distribute additional ADSs representing any shares we distribute as a dividend or free distribution. The depositary
will only distribute whole ADSs. It will sell shares which would require it to deliver a fraction of an ADS (or ADSs representing
those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional
ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed shares sufficient
to pay its fees and expenses in connection with that distribution (or ADSs representing those shares).
Rights to purchase
additional shares.
If we offer holders of our securities any rights to subscribe for additional shares or any other rights,
the depositary may make these rights available to ADS holders. If the depositary decides it is not legal and practical to make
the rights available but that it is practical to sell the rights, the depositary will use reasonable efforts to sell the rights
and distribute the proceeds in the same way as it does with cash. The depositary will allow rights that are not distributed or
sold to lapse.
In that case, you will receive no value for them.
If the depositary makes
rights available to ADS holders, it will exercise the rights and purchase the shares on your behalf. The depositary will then deposit
the shares and deliver ADSs to the persons entitled to them. It will only exercise rights if you pay it the exercise price and
any other charges the rights require you to pay.
U.S. securities laws
may restrict transfers and cancellation of the ADSs represented by shares purchased upon exercise of rights. For example, you may
not be able to trade these ADSs freely in the United States. In this case, the depositary may deliver restricted depositary shares
that have the same terms as the ADSs described in this section except for changes needed to put the necessary restrictions in place.
Other Distributions.
The depositary will send to ADS holders anything else we distribute on deposited securities by any means it thinks is legal, fair
and practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide to sell what we distributed
and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what we distributed, in which
case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities
(other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is legal to make that distribution. The
depositary may sell a portion of the distributed securities or property sufficient to pay its fees and expenses in connection with
that distribution.
The depositary is not
responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation
to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other
action to permit the distribution of ADSs, shares, rights or anything else to ADS holders.
This means that you may not receive
the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available to
you
.
Deposit, Withdrawal
and Cancellation
How are ADSs issued?
The depositary will
deliver ADSs if you or your broker deposits shares or evidence of rights to receive shares with the custodian. Upon payment of
its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register
the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons
that made the deposit.
How can ADS holders
withdraw the deposited securities?
You may surrender your
ADSs at the depositary’s office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or
stock transfer taxes or fees, the depositary will deliver the shares and any other deposited securities underlying the ADSs
to the ADS holder or a person the ADS holder designates at the office of the custodian. Or, at your request, risk and
expense, the depositary will deliver the deposited securities at its office, if feasible.
How do ADS
holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your
ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will
send to the ADS holder a statement confirming that the ADS holder is the registered holder of uncertificated ADSs. Alternatively,
upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs requesting the exchange
of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to the ADS holder an ADR evidencing those
ADSs.
Voting Rights
How do you vote?
ADS holders may instruct
the depositary how to vote the number of deposited shares their ADSs represent. The depositary will notify ADS holders of shareholders’
meetings and arrange to deliver our voting materials to them if we ask it to. Those materials will describe the matters to be voted
on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they much reach the depositary
by a date set by the depositary.
Otherwise, you won’t be able to exercise your right to vote unless you withdraw
the shares. However, you may not know about the meeting enough in advance to withdraw the shares.
The depositary will
try, as far as practical, subject to the laws of Israel, and of our articles of association or similar documents, to vote or to
have its agents vote the shares or other deposited securities as instructed by ADS holders. The depositary will only vote or attempt
to vote as instructed.
We cannot assure you
that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. In addition,
the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out
voting instructions.
This means that you may not be able to exercise your right to vote and there may be nothing
you can do if your shares are not voted as you requested.
In order to give you
a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to deposited securities, if we
request the depositary to act, we agree to give the depositary notice of any such meeting and details concerning the matters to
be voted upon at least 30 days in advance of the meeting date.
Fees and Expenses
|
Persons depositing or withdrawing shares or ADS holders must pay
:
|
|
|
|
For
:
|
|
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs)
|
|
|
●
|
Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property
|
|
|
|
|
●
|
Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates
|
|
$.05 (or less) per ADS
|
|
|
●
|
Any cash distribution to ADS holders
|
|
A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs
|
|
|
●
|
Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to ADS holders
|
|
$.05 (or less) per ADS per calendar year
|
|
|
●
|
Depositary services
|
|
Registration or transfer fees
|
|
|
●
|
Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares
|
|
Expenses of the depositary
|
|
|
●
|
Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement)
|
|
|
|
|
●
|
converting foreign currency to U.S. dollars
|
|
Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes
|
|
|
●
|
As necessary
|
|
Any charges incurred by the depositary or its agents for servicing the deposited securities
|
|
|
●
|
As necessary
|
The depositary collects
its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal
or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees
from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its
annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry
system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution
payable to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services
until its fees for those services are paid.
From time to time, the
depositary may make payments to us to reimburse and/or share revenue from the fees collected from ADS holders, or waive fees and
expenses for services provided, generally relating to costs and expenses arising out of establishment and maintenance of the ADS
program. In performing its duties under the deposit agreement, the depositary may use brokers, dealers or other service providers
that are affiliates of the depositary and that may earn or share fees or commissions.
Payment of Taxes
You will be responsible
for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs.
The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented by
your ADSs until such taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented
by your ADSs to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities,
it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders
any property, remaining after it has paid the taxes.
Reclassifications, Recapitalizations
and Mergers
|
If we:
|
|
Then:
|
|
●
|
Change the nominal or par value of our shares
|
|
The cash, shares or other securities received by the depositary
will become deposited securities. Each ADS will automatically represent its equal share of the new deposited securities.
The depositary may distribute new ADSs representing the new
deposited securities or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities.
|
|
●
|
Reclassify, split up or consolidate any of the deposited securities
|
|
|
●
|
Distribute securities on the shares that are not distributed to you
|
|
|
●
|
Recapitalize, reorganize, merge, liquidate, sell all or substantially all of our assets, or take any similar action
|
|
Amendment and Termination
How may the deposit
agreement be amended?
We may agree with the
depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or increases fees
or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs,
delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding
ADSs until 30 days after the depositary notifies ADS holders of the amendment.
At the time an amendment becomes effective, you
are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement
as amended
.
How may the deposit
agreement be terminated?
The depositary will terminate the deposit agreement at our direction by mailing notice of termination
to the ADS holders then outstanding at least 30 days prior to the date fixed in such notice for such termination. The depositary
may also terminate the deposit agreement by mailing notice of termination to us and the ADS holders if 60 days have passed since the
depositary told us it wants to resign but a successor depositary has not been appointed and accepted its appointment.
After termination, the
depositary and its agents will do the following under the deposit agreement but nothing else: collect distributions on the deposited
securities, sell rights and other property, and deliver shares and other deposited securities upon cancellation of ADSs. Four months
after termination, the depositary may sell any remaining deposited securities by public or private sale. After that,
the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement
for the pro rata benefit of the ADS holders that have not surrendered their ADSs. It will not invest the money and has no liability
for interest. The depositary’s only obligations will be to account for the money and other cash. After termination our only
obligations will be to indemnify the depositary and to pay fees and expenses of the depositary that we agreed to pay.
Limitations on Obligations
and Liability
Limits on our Obligations
and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement
expressly limits our obligations and the obligations of the depositary. It also limits our liability and the liability
of the depositary. We and the depositary:
|
|
●
|
are only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith;
|
|
|
|
|
|
|
●
|
are not liable if we are or it is prevented or delayed by law or circumstances beyond our or its control from performing our or its obligations under the deposit agreement;
|
|
|
|
|
|
|
●
|
are not liable if we or it exercises discretion permitted under the deposit agreement;
|
|
|
|
|
|
|
●
|
are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement, or for any special, consequential or punitive damages for any breach of the terms of the deposit agreement;
|
|
|
|
|
|
|
●
|
have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf of any other person;
|
|
|
|
|
|
|
●
|
are not liable for the acts or omissions of any securities depository, clearing agency or settlement system; and
|
|
|
|
|
|
|
●
|
may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person.
|
In the deposit agreement,
we and the depositary agree to indemnify each other under certain circumstances.
Requirements for
Depositary Actions
Before the depositary
will deliver or register a transfer of ADSs, make a distribution on ADSs, or permit withdrawal of shares, the depositary may require:
|
|
●
|
payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities;
|
|
|
|
|
|
|
●
|
satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and
|
|
|
|
|
|
|
●
|
compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents.
|
The depositary may refuse
to deliver ADSs or register transfers of ADSs when the transfer books of the depositary or our transfer books are closed or at
any time if the depositary or we think it advisable to do so.
Right to Receive the
Shares Underlying your ADSs
ADS holders have the
right to cancel their ADSs and withdraw the underlying shares at any time except:
|
|
●
|
when
temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books; (ii)
the transfer of shares is blocked to permit voting at a shareholders’ meeting; or (iii) we are paying a dividend on our
shares;
|
|
|
|
|
|
|
●
|
when you owe money to pay fees, taxes and similar charges; or
|
|
|
|
|
|
|
●
|
when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of shares or other deposited securities.
|
This right of withdrawal
may not be limited by any other provision of the deposit agreement.
Pre-release of ADSs
The deposit
agreement permits the depositary to deliver ADSs before deposit of the underlying shares. This is called a pre-release of the
ADSs. The depositary may also deliver shares upon cancellation of pre-released ADSs (even if the ADSs are canceled before the
pre-release transaction has been closed out). A pre-release is closed out as soon as the underlying shares are delivered to
the depositary. The depositary may receive ADSs instead of shares to close out a pre-release. The depositary may pre-release
ADSs only under the following conditions: (1) before or at the time of the pre-release, the person to whom the pre-release is
being made represents to the depositary in writing that it or its customer owns the shares or ADSs to be deposited; (2) the
pre-release is fully collateralized with cash or other collateral that the depositary considers appropriate; and (3) the
depositary must be able to close out the pre-release on not more than five business days’ notice. In addition, the
depositary will limit the number of ADSs that may be outstanding at any time as a result of pre-release, although the
depositary may disregard the limit from time to time if it thinks it is appropriate to do so.
Direct Registration
System
In the deposit agreement,
all parties to the deposit agreement acknowledge that the Direct Registration System, or DRS, and Profile Modification System,
or Profile, will apply to uncertificated ADSs upon acceptance thereof to DRS by the Depository Trust Company, or DTC. DRS is the
system administered by DTC that facilitates interchange between registered holding of uncertificated ADSs and holding of security
entitlements in ADSs through DTC and a DTC participant. Profile is a required feature of DRS that allows a DTC participant, claiming
to act on behalf of a registered holder of ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee
and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from
the ADS holder to register that transfer.
In connection with
and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement understand
that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder in
requesting registration of transfer and delivery described in the paragraph above has the actual authority to act on behalf of
the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree
that the depositary’s reliance on and compliance with instructions received by the depositary through the DRS/Profile System
and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder communications;
inspection of register of holders of ADSs
The depositary will
make available for your inspection at its office all communications that it receives from us as a holder of deposited securities
that we make generally available to holders of deposited securities. The depositary will send you copies of those communications
if we ask it to. You have a right to inspect the register of holders of ADSs, but not for the purpose of contacting those holders
about a matter unrelated to our business or the ADSs.
DESCRIPTION OF WARRANTS
We may issue warrants
to purchase ADSs and/or ordinary shares. Warrants may be issued independently or together with any other securities and may be
attached to, or separate from, such securities. Each series of warrants will be issued under a separate warrant agreement to be
entered into between us and a warrant agent. The warrant agent will act solely as our agent and will not assume any obligation
or relationship of agency for or with holders or beneficial owners of warrants. The terms of any warrants to be issued and a description
of the material provisions of the applicable warrant agreement will be set forth in the applicable prospectus supplement.
The applicable prospectus
supplement will describe the following terms of any warrants in respect of which this prospectus is being delivered:
|
|
●
|
the
title of such warrants;
|
|
|
|
|
|
|
●
|
the aggregate number of such warrants;
|
|
|
|
|
|
|
●
|
the price or prices at which such warrants will be issued and exercised;
|
|
|
|
|
|
|
●
|
the currency or currencies in which the price of such warrants will be payable;
|
|
|
|
|
|
|
●
|
the securities purchasable upon exercise of such warrants;
|
|
|
|
|
|
|
●
|
the date on which the right to exercise such warrants shall commence and the date on which such right shall expire;
|
|
|
|
|
|
|
●
|
if applicable, the minimum or maximum amount of such warrants which may be exercised at any one time;
|
|
|
|
|
|
|
●
|
if applicable, the designation and terms of the securities with which such warrants are issued and the number of such warrants issued with each such security;
|
|
|
|
|
|
|
●
|
if applicable, the date on and after which such warrants and the related securities will be separately transferable;
|
|
|
|
|
|
|
●
|
information with respect to book-entry procedures, if any;
|
|
|
|
|
|
|
●
|
any material Israeli and United States federal income tax consequences;
|
|
|
|
|
|
|
●
|
the anti-dilution provisions of the warrants, if any; and
|
|
|
|
|
|
|
●
|
any other terms of such warrants, including terms, procedures and limitations relating to the exchange and exercise of such warrants.
|
The description
in the applicable prospectus supplement of any warrants we offer will not necessarily be complete and will be qualified in its
entirety by reference to the applicable warrant agreement, which will be filed with the SEC if we offer warrants. For more information
on how you can obtain copies of the applicable warrant agreement if we offer warrants, see “Where You Can Find More Information;
Incorporation of Information by Reference” beginning on page 20. We urge you to read the applicable warrant agreement and
any applicable prospectus supplement in their entirety.
DESCRIPTION OF SUBSCRIPTION
RIGHTS
We may issue subscription
rights to purchase our ordinary shares and/or our ADSs. These subscription rights may be issued independently or together with
any other security offered hereby and may or may not be transferable by the shareholder receiving the subscription rights in such
offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters
or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining
unsubscribed for after such offering.
The prospectus
supplement relating to any subscription rights we offer, if any, will, to the extent applicable, include specific terms relating
to the offering, including some or all of the following:
|
|
●
|
the price, if any, for the subscription rights;
|
|
|
|
|
|
|
●
|
the exercise price payable for each ordinary share and/or ADS upon the exercise of the subscription rights;
|
|
|
|
|
|
|
●
|
the number of subscription rights to be issued to each shareholder;
|
|
|
|
|
|
|
●
|
the number and terms of the ordinary shares and/or ADSs which may be purchased per each subscription right;
|
|
|
|
|
|
|
●
|
the extent to which the subscription rights are transferable;
|
|
|
|
|
|
|
●
|
any other terms of the subscription rights, including the terms, procedures and limitations relating to the exchange and exercise of the subscription rights;
|
|
|
|
|
|
|
●
|
the date on which the right to exercise the subscription rights shall commence, and the date on which the subscription rights shall expire;
|
|
|
|
|
|
|
●
|
the extent to which the subscription rights may include an over-subscription privilege with respect to unsubscribed securities; and
|
|
|
|
|
|
|
●
|
if applicable, the material terms of any standby underwriting or purchase arrangement which may be entered into by us in connection with the offering of subscription rights.
|
The description in
the applicable prospectus supplement of any subscription rights we offer will not necessarily be complete and will be qualified
in its entirety by reference to the applicable subscription right agreement, which will be filed with the SEC if we offer subscription
rights. For more information on how you can obtain copies of the applicable subscription right agreement if we offer subscription
rights, see “Where You Can Find More Information; Incorporation of Information by Reference” beginning on page 20.
We urge you to read the applicable subscription right agreement and any applicable prospectus supplement in their entirety.
DESCRIPTION OF UNITS
We may issue units
comprised of one or more of the other securities that may be offered under this prospectus, in any combination. Each unit will
be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will
have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide
that the securities included in the unit may not be held or transferred separately at any time, or at any time before a specified
date.
The prospectus supplement
relating to any units we offer, if any, will, to the extent applicable, include specific terms relating to the offering, including
some or all of the following:
|
|
●
|
the material terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
|
|
|
|
|
|
|
●
|
any material provisions relating to the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and
|
|
|
|
|
|
|
●
|
any material provisions of the governing unit agreement that differ from those described above.
|
The description in
the applicable prospectus supplement of any units we offer will not necessarily be complete and will be qualified in its entirety
by reference to the applicable unit agreement, which will be filed with the SEC if we offer units. For more information on how
you can obtain copies of the applicable unit agreement if we offer units, see “Where You Can Find More Information; Incorporation
of Information by Reference” beginning on page 20. We urge you to read the applicable unit agreement and any applicable prospectus
supplement in their entirety.
PLAN OF DISTRIBUTION
The securities being
offered by this prospectus may be sold:
|
|
●
|
through agents;
|
|
|
|
|
|
|
●
|
to or through one or more underwriters on a firm commitment or agency basis;
|
|
|
|
|
|
|
●
|
through put or call option transactions relating to the securities;
|
|
|
|
|
|
|
●
|
through broker-dealers;
|
|
|
|
|
|
|
●
|
directly to purchasers, through a specific bidding or auction process, on a negotiated basis or otherwise;
|
|
|
|
|
|
|
●
|
through any other method permitted pursuant to applicable law; or
|
|
|
|
|
|
|
●
|
through a combination of any such methods of sale.
|
At any time a particular
offer of the securities covered by this prospectus is made, a revised prospectus or prospectus supplement, if required, will be
distributed which will set forth the aggregate amount of securities covered by this prospectus being offered and the terms of the
offering, including the name or names of any underwriters, dealers, brokers or agents, any discounts, commissions, concessions
and other items constituting compensation from us and any discounts, commissions or concessions allowed or re-allowed or paid to
dealers. Such prospectus supplement, and, if necessary, a post-effective amendment to the registration statement of which this
prospectus is a part, will be filed with the SEC to reflect the disclosure of additional information with respect to the distribution
of the securities covered by this prospectus. In order to comply with the securities laws of certain states, if applicable, the
securities sold under this prospectus may only be sold through registered or licensed broker-dealers. In addition, in some states
the securities may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from
registration or qualification requirements is available and is complied with.
The distribution of
securities may be effected from time to time in one or more transactions, including block transactions and transactions on the
Nasdaq Capital Market or any other organized market where the securities may be traded. The securities may be sold at a fixed price
or prices, which may be changed, or at market prices prevailing at the time of sale, at prices relating to the prevailing market
prices or at negotiated prices. The consideration may be cash or another form negotiated by the parties. Agents, underwriters or
broker-dealers may be paid compensation for offering and selling the securities. That compensation may be in the form of discounts,
concessions or commissions to be received from us or from the purchasers of the securities. Any dealers and agents participating
in the distribution of the securities may be deemed to be underwriters, and compensation received by them on resale of the securities
may be deemed to be underwriting discounts. If any such dealers or agents were deemed to be underwriters, they may be subject to
statutory liabilities under the Securities Act of 1933, as amended, or the Securities Act.
Agents may from time
to time solicit offers to purchase the securities. If required, we will name in the applicable prospectus supplement any agent
involved in the offer or sale of the securities and set forth any compensation payable to the agent. Unless otherwise indicated
in the prospectus supplement, any agent will be acting on a best efforts basis for the period of its appointment. Any agent selling
the securities covered by this prospectus may be deemed to be an underwriter, as that term is defined in the Securities Act, of
the securities.
If underwriters are
used in a sale, securities will be acquired by the underwriters for their own account and may be resold from time to time in one
or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the
time of sale, or under delayed delivery contracts or other contractual commitments. Securities may be offered to the public either
through underwriting syndicates represented by one or more managing underwriters or directly by one or more firms acting as underwriters.
If an underwriter or underwriters are used in the sale of securities, an underwriting agreement will be executed with the underwriter
or underwriters, as well as any other underwriter or underwriters, with respect to a particular underwritten offering of securities,
and will set forth the terms of the transactions, including compensation of the underwriters and dealers and the public offering
price, if applicable. The prospectus and prospectus supplement will be used by the underwriters to resell the securities.
If a dealer is used
in the sale of the securities, we or an underwriter will sell the securities to the dealer, as principal. The dealer may then resell
the securities to the public at varying prices to be determined by the dealer at the time of resale. To the extent required, we
will set forth in the prospectus supplement the name of the dealer and the terms of the transactions.
We may directly solicit
offers to purchase the securities and may make sales of securities directly to institutional investors or others. These persons
may be deemed to be underwriters within the meaning of the Securities Act with respect to any resale of the securities. To the
extent required, the prospectus supplement will describe the terms of any such sales, including the terms of any bidding or auction
process, if used.
Agents, underwriters
and dealers may be entitled under agreements which may be entered into with us to indemnification by us against specified
liabilities, including liabilities incurred under the Securities Act, or to contribution by us to payments they may be required
to make in respect of such liabilities. If required, the prospectus supplement will describe the terms and conditions of the indemnification
or contribution. Some of the agents, underwriters or dealers, or their affiliates may be customers of, engage in transactions with
or perform services for us or our subsidiaries.
Any person participating
in the distribution of securities registered under the registration statement that includes this prospectus will be subject to
applicable provisions of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the applicable SEC rules and
regulations, including, among others, Regulation M, which may limit the timing of purchases and sales of any of our securities
by that person. Furthermore, Regulation M may restrict the ability of any person engaged in the distribution of our securities
to engage in market-making activities with respect to our securities. These restrictions may affect the marketability of our securities
and the ability of any person or entity to engage in market-making activities with respect to our securities.
Certain persons participating
in an offering may engage in over-allotment, stabilizing transactions, short-covering transactions, penalty bids and other transactions
that stabilize, maintain or otherwise affect the price of the offered securities. These activities may maintain the price of the
offered securities at levels above those that might otherwise prevail in the open market, including by entering stabilizing bids,
effecting syndicate covering transactions or imposing penalty bids, each of which is described below.
|
|
●
|
A stabilizing bid means the placing of any bid, or the effecting of any purchase, for the purpose of pegging, fixing or maintaining the price of a security.
|
|
|
|
|
|
|
●
|
A syndicate covering transaction means the placing of any bid on behalf of the underwriting syndicate or the effecting of any purchase to reduce a short position created in connection with the offering.
|
|
|
|
|
|
|
●
|
A penalty bid means an arrangement that permits the managing underwriter to reclaim a selling concession from a syndicate member in connection with the offering when offered securities originally sold by the syndicate member are purchased in syndicate covering transactions.
|
These transactions may
be effected on an exchange or automated quotation system, if the securities are listed on that exchange or admitted for trading
on that automated quotation system, or in the over-the-counter market or otherwise.
If so indicated in the
applicable prospectus supplement, we will authorize agents, underwriters or dealers to solicit offers from certain types of institutions
to purchase offered securities from us at the public offering price set forth in such prospectus supplement pursuant to delayed
delivery contracts providing for payment and delivery on a specified date in the future. Such contracts will be subject only to
those conditions set forth in the prospectus supplement and the prospectus supplement will set forth the commission payable for
solicitation of such contracts.
In addition, ordinary
shares or ADSs may be issued upon conversion of or in exchange for debt securities or other securities.
Any underwriters to
whom offered securities are sold for public offering and sale may make a market in such offered securities, but such underwriters
will not be obligated to do so and may discontinue any market making at any time without notice. The offered securities may or
may not be listed on a national securities exchange. No assurance can be given that there will be a market for the offered securities.
Any securities that
qualify for sale pursuant to Rule 144 or Regulation S under the Securities Act, may be sold under Rule 144 or Regulation S rather
than pursuant to this prospectus.
To the extent that
we make sales to or through one or more underwriters or agents in at-the-market offerings, we will do so pursuant to the terms
of a distribution agreement between us and the underwriters or agents. If we engage in at-the-market sales pursuant to a distribution
agreement, we will sell our ordinary shares or ADSs to or through one or more underwriters or agents, which may act on an agency
basis or on a principal basis. During the term of any such agreement, we may sell ordinary shares or ADSs on a daily basis in
exchange transactions or otherwise as we agree with the underwriters or agents. The distribution agreement will provide that any
ordinary shares or ADSs sold will be sold at prices related to the then prevailing market prices for our ordinary shares or ADSs.
Therefore, exact figures regarding proceeds that will be raised or commissions to be paid cannot be determined at this time and
will be described in a prospectus supplement. Pursuant to the terms of the distribution agreement, we also may agree to sell,
and the relevant underwriters or agents may agree to solicit offers to purchase, blocks of our ordinary shares, ADSs or warrants.
The terms of each such distribution agreement will be set forth in more detail in a prospectus supplement to this prospectus.
In connection with offerings
made through underwriters or agents, we may enter into agreements with such underwriters or agents pursuant to which we receive
our outstanding securities in consideration for the securities being offered to the public for cash. In connection with these arrangements,
the underwriters or agents may also sell securities covered by this prospectus to hedge their positions in these outstanding securities,
including in short sale transactions. If so, the underwriters or agents may use the securities received from us under these arrangements
to close out any related open borrowings of securities.
We may enter into derivative
transactions with third parties or sell securities not covered by this prospectus to third parties in privately negotiated transactions.
If the applicable prospectus supplement indicates, in connection with those derivatives, such third parties (or affiliates of such
third parties) may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale
transactions. If so, such third parties (or affiliates of such third parties) may use securities pledged by us or borrowed from
us or others to settle those sales or to close out any related open borrowings of shares, and may use securities received from
us in settlement of those derivatives to close out any related open borrowings of shares. The third parties (or affiliates of such
third parties) in such sale transactions will be underwriters and will be identified in the applicable prospectus supplement (or
a post-effective amendment).
We may loan or pledge
securities to a financial institution or other third party that in turn may sell the securities using this prospectus. Such financial
institution or third party may transfer its short position to investors in our securities or in connection with a simultaneous
offering of other securities offered by this prospectus or in connection with a simultaneous offering of other securities offered
by this prospectus.
LEGAL MATTERS
Certain legal matters
with respect to Israeli law and with respect to the validity of the offered securities under Israeli law will be passed upon for
us by Gross, Kleinhendler, Hodak, Halevy, Greenberg & Co., Tel Aviv, Israel.
Certain legal matters with respect
to U.S. federal securities law will be passed upon for us by Zysman, Aharoni, Gayer and Sullivan & Worcester LLP, New York,
New York.
EXPERTS
The financial statements
incorporated in this prospectus by reference to the Annual Report on Form 20-F for the year ended December 31, 2015 have, been
so incorporated in reliance on the report (which contains an explanatory paragraph relating to the Company’s ability to continue
as a going concern as described in Note 1d to the financial statements) of Kesselman & Kesselman, Certified Public Accountants
(Isr.), a member firm of PricewaterhouseCoopers International Limited, an independent registered public accounting firm, given
on the authority of such firm as experts in auditing and accounting.
WHERE YOU CAN FIND
MORE INFORMATION
We have filed with the
SEC a registration statement on Form F-3 under the Securities Act, with respect to the securities offered by this prospectus. However,
as is permitted by the rules and regulations of the SEC, this prospectus, which is part of our registration statement on Form F-3,
omits certain non-material information, exhibits, schedules and undertakings set forth in the registration statement. For further
information about us, and the securities offered by this prospectus, please refer to the registration statement.
We are subject to the
reporting requirements of the Exchange Act that are applicable to a foreign private issuer. In accordance with the Exchange Act,
we file reports, including annual reports on Form 20-F. We also furnish to the SEC under cover of Form 6-K material information
required to be made public in Israel, filed with and made public by any stock exchange or distributed by us to our shareholders.
The
registration statement on Form F-3 of which this prospectus forms a part, including the exhibits and schedules thereto, and reports
and other information filed by us with the SEC may be inspected without charge and copied at prescribed rates at the SEC’s
Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Copies of this material are also available by mail from the
Public Reference Section of the SEC, at 100 F. Street, N.E., Washington D.C. 20549, at prescribed rates. The public may obtain
information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site
that contains reports, proxy and information statements, and other information regarding issuers, such as us, that file electronically
with the SEC (
http://www.sec.gov
).
As
a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements
to shareholders and our officers, directors and principal shareholders are exempt from the “short-swing profits” reporting
and liability provisions contained in Section 16 of the Exchange Act and related Exchange Act rules.
INCORPORATION OF CERTAIN
DOCUMENTS BY REFERENCE
We
file annual and special reports and other information with the SEC (File Number 001-35464). These filings contain important information
which does not appear in this prospectus. The SEC allows us to “incorporate by reference” information into this prospectus,
which means that we can disclose important information to you by referring you to other documents which we have filed or will file
with the SEC. We are incorporating by reference in this prospectus the documents listed below and all amendments or supplements
we may file to such documents, as well as any future filings we may make with the SEC on Form 20-F under the Exchange Act before
the time that all of the securities offered by this prospectus have been sold or de-registered:
|
|
●
|
The description of our ordinary shares, par value NIS 0.10 per share, and the American Depositary Shares representing the ordinary shares, contained in our Registration Statement on Form 20-F filed with the SEC on May 7, 2015;
|
|
|
|
|
|
|
●
|
our Annual Report on Form 20-F for the fiscal year ended on December 31, 2015, filed with the SEC on March 30, 2016; and
|
|
|
|
|
|
|
●
|
our reports on Form 6-K furnished to the SEC on May 25, 2016, May 31, 2016 (solely with respect to Exhibits 99.2 and 99.3 therein), June 14, 2016, June 24, 2016, July 27, 2016, August 15, 2016 and August 22, 2016.
|
In addition, any reports
on Form 6-K submitted to the SEC by the registrant pursuant to the Exchange Act after the date of the initial registration statement
and prior to effectiveness of the registration statement that we specifically identify in such forms as being incorporated by reference
into the registration statement of which this prospectus forms a part and all subsequent annual reports on Form 20-F filed after
the effective date of this registration statement and prior to the termination of this offering and any reports on Form 6-K subsequently
submitted to the SEC or portions thereof that we specifically identify in such forms as being incorporated by reference into the
registration statement of which this prospectus forms a part, shall be considered to be incorporated into this prospectus by reference
and shall be considered a part of this prospectus from the date of filing or submission of such documents.
Certain statements in
and portions of this prospectus update and replace information in the above listed documents incorporated by reference. Likewise,
statements in or portions of a future document incorporated by reference in this prospectus may update and replace statements in
and portions of this prospectus or the above listed documents.
We will provide you
without charge, upon your written or oral request, a copy of any of the documents incorporated by reference in this prospectus,
other than exhibits to such documents which are not specifically incorporated by reference into such documents. Please direct
your written or telephone requests to Medigus Ltd., Omer Industrial Park, No. 7A, P.O. Box 3030, Omer 8496500, Israel,
Attn: Gilad Mamlok, telephone number +972 (8) 646-6880. You may also obtain information about us by visiting our website at
www.medigus.com
.
Information contained in our website is not part of this prospectus.
ENFORCEABILITY OF CIVIL
LIABILITIES
We are incorporated
under the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli experts named
in this prospectus, substantially all of whom reside outside the United States, may be difficult to obtain within the United States.
Furthermore, because substantially all of our assets and substantially all of our directors and officers are located outside the
United States, any judgment obtained in the United States against us or any of our directors and officers may not be collectible
within the United States.
It may be difficult
to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based
on a violation of U.S. securities laws because Israel is not the most appropriate forum to bring such a claim. In addition,
even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim.
If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact which can be a time-consuming
and costly process. Certain matters of procedure will also be governed by Israeli law.
Subject to specified
time limitations and legal procedures, Israeli courts may enforce a United States judgment in a civil matter which, subject to
certain exceptions, is non-appealable, including judgments based upon the civil liability provisions of the Securities Act and
the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that:
|
|
●
|
the judgments are obtained after due process before a court of competent jurisdiction, according to the laws of the state in which the judgment is given and the rules of private international law currently prevailing in Israel;
|
|
|
|
|
|
|
●
|
the prevailing law of the foreign state in which the judgments were rendered allows the enforcement of judgments of Israeli courts (however, the Israeli courts may waive this requirement following a request by the attorney general);
|
|
|
|
|
|
|
●
|
adequate service of process has been effected and the defendant has had a reasonable opportunity to be heard and to present his or her evidence;
|
|
|
|
|
|
|
●
|
the judgments are not contrary to public policy, and the enforcement of the civil liabilities set forth in the judgment does not impair the security or sovereignty of the State of Israel;
|
|
|
|
|
|
|
●
|
the judgments were not obtained by fraud and do not conflict with any other valid judgment in the same matter between the same parties;
|
|
|
|
|
|
|
●
|
an action between the same parties in the same matter is not pending in any Israeli court at the time the lawsuit is instituted in the foreign court; and
|
|
|
|
|
|
|
●
|
the obligations under the judgment are enforceable according to the laws of the State of Israel and according to the law of the foreign state in which the relief was granted.
|
We have irrevocably
appointed Medigus USA LLC
as our agent to receive service of process in any action against us in any United States
federal or state court arising out of this offering or any purchase or sale of securities in connection with this offering.
If a foreign judgment
is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli
currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli
currency is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange in
force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount
of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus
interest at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk
of unfavorable exchange rates.
OFFERING EXPENSES
The following is a
statement of expenses in connection with the distribution of the securities registered. All amounts shown are estimates except
the SEC registration fee. The estimates do not include expenses related to offerings of particular securities. Each prospectus
supplement describing an offering of securities will reflect the estimated expenses related to the offering of securities under
that prospectus supplement.
|
SEC registration fees
|
|
$
|
2,014
|
|
|
Legal fees and expenses
|
|
|
10,000
|
|
|
Accountants fees and expenses
|
|
|
12,000
|
|
|
Miscellaneous
|
|
|
500
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
24,514
|
|

MEDIGUS
LTD.
810,000
American Depositary Shares Representing 40,500,000 Ordinary Shares
PROSPECTUS
SUPPLEMENT
November
24, 2017
Medigus (NASDAQ:MDGS)
Historical Stock Chart
From Mar 2024 to Apr 2024
Medigus (NASDAQ:MDGS)
Historical Stock Chart
From Apr 2023 to Apr 2024