Report of Foreign Issuer (6-k)
June 22 2017 - 4:35PM
Edgar (US Regulatory)
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
6-K
REPORT OF
FOREIGN PRIVATE ISSUER
Pursuant to Rule
13a-16
or
15d-16
of the Securities Exchange Act of 1934
For the month of June 2017
Commission File Number:
001-36581
Vascular Biogenics Ltd.
(Translation of registrant’s name into English)
6 Jonathan
Netanyahu St.
Or Yehuda
Israel 6037604
(Address
of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form
20-F
or Form
40-F.
Form
20-F ☒ Form
40-F ☐
Indicate by check mark if the registrant is submitting the Form
6-K
in paper as permitted by Regulation
S-T
Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form
6-K
in paper as permitted by Regulation
S-T
Rule 101(b)(7): ☐
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the
Commission pursuant to Rule
12g3-2(b)
under the Securities Exchange Act of 1934.
Yes ☐ No ☒
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule
12g3-2(b):
82-
EXPLANATORY NOTE
Attached hereto and incorporated by reference herein is the registrant’s press release issued on June 15, 2017, entitled “VBL Therapeutics
Announces Appointment of Dr. Corinne Epperly as U.S. Chief Operating Officer”. This Report of Foreign Private Issuer on Form
6-K
shall be incorporated by reference into the Company’s
registration statement on Form
F-3
(File
No. 333-207250),
filed with the Securities and Exchange Commission (the “SEC”) on October 2, 2015, to the
extent not superseded by information subsequently filed or furnished (to the extent the Company expressly states that it incorporates such furnished information by reference) by the Company under the Securities Act of 1933, as amended, or the
Securities Exchange Act of 1934, as amended.
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
VASCULAR BIOGENICS LTD.
|
|
|
|
|
|
|
Date: June 22, 2017
|
|
|
|
By:
|
|
/s/ Dror Harats
|
|
|
|
|
|
|
|
Name: Dror Harats
Title: Chief Executive Officer
|
3
VBL Therapeutics Announces Appointment of Dr. Corinne Epperly as U.S. Chief Operating Officer
|
|
•
|
|
Industry leader with extensive oncology experience joins company as
VB-111
advances toward registration
|
TEL AVIV, Israel, June 15, 2017 (GLOBE NEWSWIRE) — VBL Therapeutics (Nasdaq:VBLT) today announced the appointment of Corinne Epperly, MD,
MPH, as U.S. Chief Operating Officer. Dr. Epperly is an oncology expert with industry background in drug development, strategy, commercialization and operations. She will have key responsibilities in forming VBL’s marketing strategy and
commercialization plan for its Phase 3 candidate
VB-111
and will work with VBL’s leadership team to advance corporate strategy and U.S. activities. Dr. Epperly will report to Dr. Dror Harats,
Chief Executive Officer of VBL.
“We are delighted to welcome Dr. Epperly to VBL’s senior leadership team,” said Dror Harats, CEO
of VBL Therapeutics. “She brings strong experience in oncology drug development and commercialization, particularly in glioblastoma. Among other achievements, she has led multibillion-dollar global transactions and complex international
negotiations, as well as corporate strategy and commercial execution. Her impressive skills and industry track record will be valuable assets to VBL as we continue to advance our lead therapeutic candidate,
VB-111,
in multiple oncology indications.”
Dr. Epperly said, “I look forward to joining VBL and
making an immediate contribution at this exciting time for the company.
VBL-111
has already demonstrated great potential as an immuno-oncology agent, having generated positive clinical data and evidence of a
survival benefit in glioblastoma, ovarian cancer and thyroid cancer. I look forward to working with VBL’s senior team to help bring this drug to patients as efficiently as possible.”
Dr. Epperly joins VBL after seven years at Bristol-Myers Squibb (BMS), where she delivered results across diverse roles spanning marketing, M&A,
strategic operations and medical strategy. Most recently she was involved in leading the preparation for the commercial launches of OPDIVO
®
(nivolumab) in both hepatocellular carcinoma and in
glioblastoma. While at BMS she led U.S. immuno-oncology safety management, advancing the safety strategy with oncology stakeholders. Dr. Epperly also served as the Global Mergers & Acquisitions Lead in the Strategic Transactions Group
at BMS. Prior to joining BMS, she was a member of the Global Healthcare Investment Research team at Goldman Sachs, based in London, where she helped relaunch the European Pharmaceutical and Biotech Team. Dr. Epperly holds a dual graduate
degree, an M.D. and an MPH from the University of North Carolina at Chapel Hill. She completed her medical training at the University of North Carolina Hospitals with the Department of Pediatrics. She also earned a Distinguished B.A. from the
University of Virginia where she studied biochemistry and biology. Prior to medical school she conducted biomedical research in Experimental Immunology on checkpoint inhibition and tumor suppressor genes in the National Cancer Institute, National
Institutes of Health.
About VBL
Vascular Biogenics
Ltd., operating as VBL Therapeutics, is a clinical stage biopharmaceutical company focused on the discovery, development and commercialization of
first-in-class
treatments for cancer. The Company’s lead oncology product candidate, ofranergene
obadenovec
(VB-111),
is a
first-in-class,
targeted anti-cancer gene-therapy
agent that is positioned to treat a wide range of solid tumors. It is conveniently administered as an IV infusion once every two months. It has been observed to be well-tolerated in >200 cancer patients and we have observed its efficacy signals
in an “all comers” Phase 1 trial as well as in three tumor-specific Phase 2 studies. Ofranergene obadenovec is currently being studied in a Phase 3 pivotal trial for recurrent Glioblastoma, conducted under an FDA Special Protocol
Assessment (SPA).
Forward Looking Statements
This
press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,”
“estimate,” “expect,” “goal,” “intend,” “look forward to”, “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,”
“would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the clinical development of ofranergene obadenovec
(VB-111),
including our
expectations regarding the timing of results from the GLOBE study, and its therapeutic potential and clinical results. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors
that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, and the risk that
historical clinical trial results may not be predictive of future trial results. In particular, results from our pivotal Phase 3 clinical trial of ofranergene obadenovec
(VB-111)
in rGBM may not support
approval of ofranergene obadenovec for marketing in the United States, notwithstanding the positive results seen in prior clinical experience. A further list and description of these risks, uncertainties and other risks can be found in the
Company’s regulatory filings with the U.S. Securities and Exchange Commission, including in our annual report on Form
20-F
for the year ended December 31, 2016. Existing and prospective investors are
cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. VBL Therapeutics undertakes no obligation to update or revise the information contained in this press release, whether as a result of
new information, future events or circumstances or otherwise.
OPDIVO
®
is a registered trademark
of Bristol-Myers Squibb Company
INVESTOR CONTACT:
Michael Rice
LifeSci Advisors, LLC
(646)
597-6979
MEDIA
CONTACT:
Matt Middleman, M.D.
LifeSci Public Relations
(646)
627-8384

Vascular Biogenics (NASDAQ:VBLT)
Historical Stock Chart
From Mar 2024 to Apr 2024
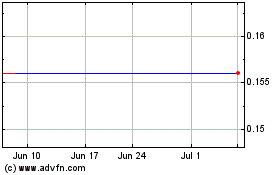
Vascular Biogenics (NASDAQ:VBLT)
Historical Stock Chart
From Apr 2023 to Apr 2024