FORM 6-K
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Report of Foreign Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the period ended March 31, 2015
Commission File Number: 001-12033
Nymox Pharmaceutical Corporation
9900 Cavendish Blvd., St. Laurent, QC, Canada, H4M 2V2
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F:
Form 20-F [ X ] Form 40-F [ ]
Indicate by check mark if the registrant is submitting Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(l): [ ]
Indicate by check mark if the registrant is submitting Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): [ ]
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes [ ] No [ X ]
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82-______________
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
NYMOX PHARMACEUTICAL CORPORATION |
|
(Registrant) |
|
|
|
|
|
By: /s/ Paul Averback, MD |
|
Paul Averback, MD |
|
President and Chief Executive Officer |
Date: May 15, 2015
Exhibit 99.1

MANAGEMENT'S DISCUSSION AND ANALYSIS (in US dollars)
This Management’s discussion and analysis (“MD&A”) comments on the Corporation’s operations, performance and financial condition as at and for the quarters ended March 31, 2015, 2014 and 2013. This MD&A should be read together with the unaudited condensed interim Consolidated Financial Statements and the related notes. This MD&A is dated May 15, 2015. All amounts in this report are in U.S. dollars, unless otherwise noted.
Except as otherwise indicated, all financial information contained in this MD&A and in the unaudited condensed interim Consolidated Financial Statements has been prepared in accordance with International Financial Reporting Standards (“IFRS”) a s issued by the International Accounting Standards Board (“IASB”). The unaudited condensed interim Consolidated Financial Statements and this MD&A were reviewed by the Corporation’s Audit Committee and were approved by our Board of Directors.
Additional information about the Corporation can be obtained on EDGAR at www.sec.gov or on SEDAR at www.sedar.com.
Overview
Corporate Profile
Nymox Pharmaceutical Corporation is a biopharmaceutical company focused on developing its drug candidate, NX-1207, for the treatment of BPH and the treatment of low-grade localized prostate cancer. Since 1989, the Corporation’s activities and resources have been directed primarily on developing certain pharmaceutical technologies. Since 2002, Nymox has been developing its novel proprietary drug candidate, NX-1207, for the treatment of benign prostatic hyperplasia (“BPH”). In December 2010, the Corporation signed a license and collaboration agreement with Recordati, a European pharmaceutical group, for the development and commercialization of NX-1207 for BPH in Europe including Russia and the CIS, the Middle East, the Maghreb area of North Africa and South Africa. After the top-line statistical failure of Nymox’s U.S. Phase 3 studies NX02-0017 and NX02-0018 at 12 months post-treatment, Recordati has terminated development and commercialization efforts for NX-1207 in the licensed territories. NX-1207 showed positive results for the treatment of BPH in Phase 1 and 2 clinical trials in the U.S. and in follow-up studies of available subjects from the completed clinical trials. In 2009, Nymox started two pivotal double blind placebo controlled Phase 3 trials for NX-1207, NX02-0017 and NX02-0018, that were conducted at investigational sites across the U.S. with a total enrollment of approximately 1,000 patients. Nymox also initiated subsequent open-label U.S. re-injection Phase 3 safety studies, NX02-0020 and NX02-0022. The NX02-0017 study completed patient enrollment and participation in December 2013 and the NX02-0018 study in May 2014. Top-line results of the Phase 3 NX02-0017 and NX02-0018 U.S. clinical trials of NX-1207 for BPH at 12 months post-treatment were not statistically significant compared to placebo. The Corporation is in the process of further data analysis and assessments of the two studies, and expects to continue its efforts to work on the development program. Nymox is also developing NX-1207 for the treatment of low-grade localized prostate cancer. A Phase 2 study of NX-1207 for low grade localized prostate cancer was started in 2012 with positive results reported in 2014. The Corporation is in the process of working towards definitive studies for this indication. The Corporation also has an extensive patent portfolio covering its marketed products, its investigational drug as well as other therapeutic and diagnostic indications. Nymox developed the AlzheimAlert™ test, which is certified with a CE Mark in Europe. Nymox developed and markets NicAlert™ and TobacAlert™; which are tests that use urine or saliva to detect use of and exposure to tobacco products. NicAlert™ has received clearance from the FDA and is also certified with a CE Mark in Europe. TobacAlert™ is the first test of its kind to accurately measure second and third hand smoke exposure in individuals.
The Corporation is subject to a number of risks, including the successful development and marketing of its technologies and its ability to finance its research and development programs and operations through the sale of its common shares. Since 2003, the Corporation has relied on the Common Stock Private Purchase Agreement (the ‘Agreement’) (referred to in note 9 (a) to the condensed interim Consolidated Financial Statements), private placements and other types of financings collaboration agreements, and revenues from product sales to fund its operations and research programs. In order to achieve its business plan and the realization of its assets and liabilities in the normal course of operations, the Corporation anticipates the need to raise a dditional debt or capital in the near term and/or achieve sales and other revenue-generating activities. Management has taken steps to reduce expenditures going forward in the short term by staff reductions, deferral of management salaries, and operational changes.
The top-line failure of the two Phase 3 studies of NX-1207 for BPH materially affects the Corporation’s current ability to fund its operations, meet its cash flow requirements, realize its assets and discharge its obligations. Under the Common Stock Private Purchase Agreement, the Corporation must adhere to general covenants in order to draw on its facility, including maintaining its stock exchange listing and registration requirements and having no material adverse effects, as defined in the Agreement, wit h respect to the business and operations of the Corporation. In the past, the Corporation has been successful in obtaining the required financing pursuant to the Agreement. As of the date of the financial statements, the Corporation has not received any communication from the counterparty in the Agreement that it will not honor the Corporation`s future draw-down notices under the Agreement or that it intends to terminate the Agreement. On April 28, 2015, the Corporation completed a drawdown of $600,000 pursuant to this Agreement.
1
Management believes that current cash balances as at March 31, 2015 and anticipated funds from product sales are not sufficient to fund substantially all of its planned business operations and research and development programs over the next year. The Corporation intends to access financing through the existing Common Stock Private Purchase Agreement and/or other sources of capital in order to fund these operations and activities over the next year.
If the purchaser does not purchase the Corporation`s common shares as provided for under the Agreement, or if the Agreement is not renewed, the Corporation will have to seek other sources of financing in order to be able to pay its obligations as they become due, which could have an impact on its ability to continue as a going concern. Considering recent developments and the need for additional financing, there exists a material uncertainty that casts substantial doubt about the Corporation’s ability to con tinue as a going concern. These financial statements do not reflect adjustments that would be necessary if the going concern assumption was not appropriate. If the going concern assumption is not appropriate, then adjustments may be necessary to the carrying value and classification of assets and liabilities and reported results of operations and such adjustments could be material.
We have incurred operating losses throughout our history. Management believes that such operating losses will continue for at least the next few years as a result of expenditures relating to research and development of our potential therapeutic products.
On April 23, 2015, the shareholders approved the transfer of the Corporation’s head office and domicile from Quebec, Canada t o the Bahamas.
Risk Factors
The business activities of the Corporation since inception have been devoted principally to research and development. Accordingly, the Corporation has had limited revenues from sales and has not been profitable to date. We refer to the Risk Factors section of our Form 20-F filed on EDGAR and on SEDAR for a discussion of the management and investment issues that affect the Corporation and our industry. The risk factors that could have an impact on the Corporation’s financial results are summarize d as follows:
-
Our Clinical Trials for Our Therapeutic Products in Development, Such as NX-1207, May Not be Successful and We May Not Receive the Required Regulatory Approvals Necessary to Commercialize These Products
-
Our Clinical Trials for Certain Of Our Therapeutic Products May Be Delayed, Making it Impossible to Achieve Anticipated Development or Commercialization Timelines And Our Development of NX-1207 Has Been Delayed Due to Negative Results In Phase III Clinical Trials
-
A Setback in Any of Our Clinical Trials Would Likely Cause a Drop in the Price of our Shares
-
We May Not be Able to Make Adequate Arrangements with Third Parties for the Commercialization of our Product Candidates, such as NX-1207
-
We May Not Achieve our Projected Development Goals in the Time Frames We Announce and Expect
-
Even If We Obtain Regulatory Approvals for Our Product Candidates, We Will be Subject to Stringent Ongoing Government Regulation
-
It is Uncertain When, if Ever, We Will Make a Profit
-
We Will Require Additional Funding to Continue as a Going Concern
-
Our Ability to Draw on the Common Stock Private Purchase Agreement, Which Expires in November 2015, is Dependent on Adhering to General Covenants
-
We Have Identified a Material Weakness in our Internal Control over Financial Reporting. Although We Expect to Make Every Effort to Address this Material Weakness, We May Find that We are Unable to Remediate this Deficiency in our Control Environment, Which Could Reduce the Reliability of Our Financial Reporting, Harm Investor Confidence in our Company and Affect the Value of our Common Stock.
-
We Face Challenges in Developing, Manufacturing and Improving Our Products
-
Our Products and Services May Not Receive Necessary Regulatory Approvals
-
We Face Significant and Growing Competition
-
We May Not Be Able to Successfully Market Our Products
-
Protecting Our Patents and Proprietary Information is Costly and Difficult
-
We Face Changing Market Conditions
-
Health Care Plans May Not Cover or Adequately Pay for Our Products and Services
-
We Are Subject to Continuing Potential Product Liability Risks, Which Could Cost Us Material Amounts of Money
-
We Have Become Involved in Securities Class Action Litigation That is Expected to Divert Management’s Attention and Could
Harm our Business
-
The Issuance of New Shares May Dilute Nymox’s Stock
-
If We Fail to Regain Compliance With the Requirements for Continued Listing on The NASDAQ Stock Market, Our Common Shares Could be Delisted from Trading on the NASDAQ Stock Market, Which Would Adversely Affect the Liquidity of Our Common Shares and Our Ability to Raise Additional Capital
-
We Face Potential Losses Due to Foreign Currency Exchange Risks
-
We Have Never Paid a Dividend and are Unlikely to do so in the Foreseeable Future
2
Critical Accounting Policies
The condensed interim Consolidated Financial Statements of the Corporation have been prepared under International Financial Reporting Standards as issued by the International Accounting Standards Board. The Corporation’s functional and presentation currency is the United States dollar. Our accounting policies are described in the notes to our annual audited consolidated financial statements. We consider the following policies to be the most critical in understanding the judgments that are involved in preparing our financial statements and the matters that could impact our results of operations, financial condition and cash flows.
The going concern basis of presentation
The condensed interim Consolidated Financial Statements have been prepared under the going concern assumption. Refer to ‘Corporate Profile’ and note 1 to the condensed interim consolidated financial statements for a detailed discussion of this matter.
Revenue Recognition
The Corporation has generally derived its revenue from product sales and collaboration agreements. Revenue from product sales is recognized when the product has been delivered and obligations as defined in the agreement are performed. Collaboration agreements that include multiple deliverables are considered to be multiple-element arrangements. Under this type of arrangement, the identification of separate units of accounting is required and revenue is allocated among the separate units based on their relative fair values.
Payments received under a collaboration agreement may include upfront payments, milestone payments, sale of goods, royalties and license fees. Revenue for each unit of accounting are recorded as described below:
Upfront payments are deferred and recognized as revenue on a systematic basis over the estimated service period. Changes in estimates are recognized prospectively when changes to the expected term are determined.
Revenue subject to the achievement of milestones is recognized only when the specified events have occurred and collectability is reasonably assured.
Specifically, the criteria for recognizing milestone payments are that (i) the milestone is substantive in nature, (ii) the achievement was not reasonably assured at the inception of the agreement, and (iii) the Corporation has no further involvement or obligation to perform associated with the achievement of the milestone, as defined in the related collaboratio n arrangement.
Revenue from the sale of goods is recognized when the Corporation has transferred to the buyer the significant risks and rewards of ownership of the goods, there is no continuing management involvement with the goods, and the amount of revenue can be measured reliably.
| (iv) |
Royalties and license fees: |
Royalties and license fees are recognized when conditions and events under the license agreement have occurred and collectability is reasonably assured.
Revenue recognition is subject to critical judgments, particularly in the collaboration agreement described above. Managemen t uses judgment in estimating the service period over which revenue is recognized for upfront payments received.
Stock-based Compensation
Stock-based compensation is recorded using the fair value based method for stock options issued to employees and non -employees. Under this method, compensation cost related to employee awards is measured at fair value at the date of grant, net of forfeitures, and is expensed over the award’s vesting period. The Corporation uses the Black -Scholes options pricing model to calculate stock option values, which requires certain assumptions, including the future stock price volatility and expected time to exercise. There is estimation uncertainty with respect to selecting inputs to the Black-Scholes pricing model used to determine the fair value of the stock options. Changes to any of these assumptions, or the use of a different option pricing model, could produce different fair values for stock-based compensation, which could have a material impact on the Corporation’s earnings.
Contingent liabilities
Subsequent to the press release dated November 2, 2014 announcing Top-line results of the phase 3 NX02-0017 and NX02-0018 U.S. clinical trials of NX-1207 for BPH at 12 months post-treatment, a plaintiff was seeking certification of a class action suit against the Corporation and an officer of the Corporation. On March 10, 2015, we were served with a class-action lawsuit. Refer to note 13 to the condensed interim Consolidated Financial Statements. Assessing the recognition of contingent liabilities requires judgement in evaluating whether it is probable that economic benefits will be required to settle the matters subject to litigation.
3
Compound financial instruments
Compound financial instruments issued by the Corporation comprise convertible notes that can be converted to share capital at the option of the holder, and the number of shares to be issued does not vary with changes in their fair value.
The liability component of a compound financial instrument is recognized initially at the fair value of a similar liability that does not have an equity conversion option. The model used to measure the fair value of the liability component comprises estimation uncertainty in determining the interest rate applicable to a similar liability that does not have an equity conversion option. The equity component is recognized initially at the difference between the fair value of the compound financial instrument as a whole and the fair value of the liability component. Any directly attributable transaction costs are allocated to the liability and equity components in proportion to their initial carrying amounts.
Subsequent to initial recognition, the liability component of a compound financial instrument is measured at amortized cost using the effective interest method. The equity component of a compound financial instrument is not remeasured subsequent to initial recognition.
Results of Operations
|
|
|
|
|
|
|
|
|
|
| Three Months Ended March 31 |
2015 |
2014 |
2013 |
Total revenues |
$ |
2,583,920 |
|
$ |
732,564 |
|
$ |
838,646 |
|
Net income (loss) |
$ |
1,576,551 |
|
$ |
(2,592,816) |
|
$ |
(1,093,906) |
|
Income (loss) per share (basic & diluted) |
$ |
0.04 |
|
$ |
(0.07) |
|
$ |
(0.03) |
|
Total assets |
$ |
836,105 |
|
$ |
878,951 |
|
$ |
2,281,035 |
|
Non-current financial liabilities |
$ |
1,143,440 |
|
$ |
400,000 |
|
$ |
400,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quarterly Results |
Q1 – 2015 |
Q4 – 2014 |
Q3 – 2014 |
Q2 – 2014 |
Total revenues |
$ |
2,583,920 |
|
$ |
729,136 |
|
$ |
735,529 |
|
$ |
752,280 |
|
Net income (loss) |
$ |
1,576,551 |
|
$ |
(492,799) |
|
$ |
(688,206) |
|
$ |
(820,272) |
|
Income (loss) per share (basic & diluted) |
$ |
0.04 |
|
$ |
(0.02) |
|
$ |
(0.02) |
|
$ |
(0.02) |
|
| |
Q1 – 2014 |
Q4 – 2013 |
Q3 – 2013 |
Q2 – 2013 |
Total revenues |
$ |
732,564 |
|
$ |
937,490 |
|
$ |
743,288 |
|
$ |
839,586 |
|
Net loss |
$ |
(2,592,816) |
|
$ |
(1,316,921) |
|
$ |
(1,020,387) |
|
$ |
(1,477,389) |
|
Loss per share (basic & diluted) |
$ |
(0.07) |
|
$ |
(0.04) |
|
$ |
(0.03) |
|
$ |
(0.04) |
|
The revenues in 2015, 2014 and 2013 include the recognition of revenue related to the upfront payment of €10 million (US$13.1 million) received from Recordati in December 2010. The first quarter of 2015, includes $2,508,533 in revenue recognition (see note 7 of the condensed interim Consolidated Financial Statements) compared to $654,400 for the other quarters presented, which explains the increase in revenues and net income for the period ended March 31, 2015. The increase of $1,854,133 in the first quarter of 2015 is due to the fact that the initial estimated service period of five years to recognized the upfront payment was modified, in February 2015, following the announcement, by Recordati, to interrupt the European clinical trial (see note 7 of the condensed interim Consolidated Financial Statements).
The net losses during the first quarter of 2014 includes a stock compensation charge in the amount of $1,420,185 which explains the increase in net losses for that quarter compared to other quarters presented.
Results of Operations – Q1 2015 compared to Q1 2014
Net income was $1,576,551, or $0.04 per share, for the quarter ended March 31, 2015, compared to net loss of $2,592,816, or $0.07 per share, for the quarter ended March 31, 2014. The net income in 2015 is attributable to the recognition of $2,508,533 of previously deferred revenues related to the remaining upfront payment for licensing revenues received from Recordati in December 2010 recognized in the quarter ended March 31, 2015 compared to $654,400 for the quarter ended March 31, 2014. Excluding the additional $1,854,133 deferred revenue recognized as revenue, the Corporation would have incurred a net loss of $277,582 for the quarter ended March 31, 2015 compared to net loss of $2,592,816 for the same period in 2014. The decrease is mainly due to stock compensation charges of $16,551 in 2015 compared to $1,420,185 in 2014 combined to a reduction of $492,432 in clinical trial expenditures related to NX-1207 studies. The basic and diluted weighted average number of common shares outstanding for the quarter ended March 31, 2015 were 36,272,978 and 38,280,483 respectively, compared to basic and diluted weighted average number of common shares of 34,797,302 at March 31, 2014.
Revenues
Revenues from sales of goods amounted to $75,387 for the quarter ended March 31, 2015, compared with $78,164 for the quarter ended March 31, 2014. The development of therapeutic candidates and of moving therapeutic product candidates through clinical trials is a priority for the Corporation at this time. The growth of sales will become more of a priority once these candidates have reached the marketing stage. The Corporation expects that revenues will increase if and when product candidates pass clinical trials and are launched on the market.
For the quarter ended March 31, 2015, an amount of $2,508,533 was recognized as revenue relating to the upfront payment received from Recordati in December 2010 compared with $654,400 for the quarter ended March 31, 2014. The increase of $1,854,133 in the first quarter of 2015 is due to the fact that the initial estimated service period of five years to recognize the
4
upfront payment was modified, in February 2015, following the announcement, by Recordati, to interrupt the European clinical trial (see note 7 of the condensed interim Consolidated Financial Statements). Consequently, in the first quarter of 2015, the Corporation recognized, as revenue, an amount of $2,508,533 which represented the remaining deferred revenue relating to the upfront payment received from Recordati in December 2010. As at March 31, 2015, the deferred revenue related to this transaction recorded in the statement of financial position amounted to nil (as at December 31, 2014 - $2,508,533).
Research and Development
Research and development expenditures were $559,303 for the quarter ended March 31, 2015, compared with $2,061,038 for the quarter ended March 31, 2014. Research and development expenditures include costs incurred mainly for advancing Nymox’s BPH and prostate cancer product candidate NX-1207 through clinical trials. Research and development expenditures also include stock compensation charges of $1,119 for the quarter ended March 31, 2015 and $627,860 for the quarter ended March 31, 2014. On November 2, 2014, the Corporation announced that the two Phase 3 U.S. studies of NX-1207 for the treatment of BPH, NX02-0017 and NX02-0018, failed to meet their primary efficacy endpoints. For the quarter ended March 31, 2015, a decrease of $492,432 in clinical trial expenditures, combined with a decrease of $626,741 in stock compensation charges and a decrease of $313,877 in salaries and payroll related expenses explained the reduction of expenses compared to the same period in 2014. In 2015, research tax credits was nil compared to $96,412 in 2014. The decrease in 2015 is due to the fact that the U.S. BPH 12 month trials were completed in November 2014. The Corporation expects that research and development expenditures will decrease as a result of the Corporation’s U.S. BPH trial activity reduction, pending the evaluation of the data. Because of the early stage of development and the uncertainty related to the Corporation’s R&D projects, it is impossible to outline the nature, timing or estimated costs of the efforts necessary to complete these projects, nor the anticipated completion dates for these projects. The facts and circumstances indicating the uncertainties that preclude us from making a reasonable estimate of the costs and timi ng necessary to complete projects include the risks inherent in any field trials, the uncertainty as to the nature and extent of regulatory requirements both for safety and efficacy, and the ability to manufacture the products in accordance with current good manufacturing requirements (cGMP) and in sufficient quantities both for large scale trials and for commercial use as further described in the section entitled “Risk Factors”. A drug candidate that shows efficacy can take a long period (7 years or more) to achieve regulatory approval. There is also uncertainty whether we will be able to successfully adapt our patented technologies or whether any new products we develop will pass proof-of-principle testing both in the laboratory and in clinical trials, and whether we will be able to manufacture such products at a commercially competitive price. In addition, given the very high costs of development of therapeutic products, we anticipate having to partner with larger pharmaceutical companies to bring therapeutic products to market. The terms of such partnership arrangements along with our related financial obligations cannot be determined at this time and the timing of completion of the approval of such products will likely not be within our sole control.
Marketing Expenses
Marketing expenditures were $9,319 for the quarter ended March 31, 2015, compared with $42,545 for the quarter ended March 31, 2014. The decrease is mainly due to the reduction of $32,457 in salaries and payroll related expenses following staff reductions. The Corporation expects that marketing expenditures will increase if and when new products are launched on the market.
General and Administrative Expenses
General and administrative expenses were $348,799 for the quarter ended March 31, 2015, compared with $1,255,563 for the quarter ended March 31, 2014. The decrease in general and administrative expenditures is due to stock compensation charges of $15,432 for the quarter ended March 31, 2015 compared to $792,325 in the comparative period in 2014 and a decrease of $67,772 in salaries and payroll related expenses following staff reductions. The Corporation expects that general and administrative expenditures will increase as new product development leads to expanded operations.
Finance costs
Net finance costs were $52,487 for the quarter ended March 31, 2015, compared with $5,606 for the quarter ended March 31, 2014. The increase is primarily due to interest and accretion expenses of $49,339 incurred in connection with the convertible notes.
The Corporation incurs expenses in the local currency of the countries in which it operates, which include the United States and Canada. Foreign exchange fluctuations had no meaningful impact on the Corporation’s results in 2015 , 2014 or 2013.
Inflation
The Corporation does not believe that inflation has had a significant impact on its results of operations .
Results of Operations – Q1 2014 compared to Q1 2013
Net losses were $2,592,816, or $0.07 per share, for the quarter ended March 31, 2014, compared to $1,093,906, or $0.03 per share, for the quarter ended March 31, 2013. The increase in net loss is due to stock compensation charges of $1,420,185 in 2014 compared to $26,640 in 2013. The weighted average number of common shares outstanding for the quarter ended March 31, 2014 was 34,797,302 compared to 33,679,486 for the same period in 2013.
5
Revenues
Revenues from sales of goods amounted to $78,164 for the quarter ended March 31, 2014, compared with $184,246 for the quarter ended March 31, 2013. The decrease in the first quarter of 2014 compared to the same period in 2013 is due to a decrease of $33,156 in sales of NicAlert™/TobacAlert™ as well as the non-recurrence of the sale of goods of $72,926 in 2013 under our licensing agreement with Recordati. The development of therapeutic candidates and of moving therapeutic product candidates through clinical trials is a priority for the Corporation at this time. The growth of sales will become more of a priority once these candidates have reached the marketing stage. The Corporation expects that revenues will increase if and when product candidates pass clinical trials and are launched on the market.
For the quarters ended March 31, 2014 and 2013, amounts of $654,400 were recognized as revenue relating to the upfront payment received from Recordati in December 2010. At March 31, 2014, the deferred revenue related to this transaction recorded in the statement of financial position amounted to $4,471,733.
Research and Development
Research and development expenditures were $2,061,038 for the quarter ended March 31, 2014, compared with $1,471,153 for the quarter ended March 31, 2013. Research and development expenditures include costs incurred in advancing Nymox’s BPH product candidate NX-1207 through clinical trials, as well as costs related to Nymox’s localized low-grade prostate cancer clinical trials. The increase in expenses is attributable to stock compensation charges of $627,860 in the quarter ended March 31, 2014 and $4,848 in the comparative period in 2013. In 2014, research tax credits amounted to $96,412 compared to $64,377 in 2013. The Corporation expects that research and development expenditures will decrease as product candidates finish development and clinical trials. However, because of the early stage of development of the Corporation’s R&D projects, it is impossible to outline the nature, timing or estimated costs of the efforts necessary to complete these projects, nor the anticipated completion dates for these projects. The facts and circumstances indicating the uncertainties that preclude us from making a reasonable estimate of the costs and timing necessary to complete projects include the risks inherent in any field trials, the uncertainty as to the nature and extent of regulatory requirements both for safety and efficacy, and the ability to manufacture the products in accordance with current good manufacturing requirements (cGMP) and in sufficient quantities both for large scale trials and for commercial use. A drug candidate that shows efficacy can take a long period (7 years or more) to achieve regulatory approval. There is also uncertainty whether we will be able to successfully adapt our patented technologies or whether any new products we develop will pass proof -of-principle testing both in the laboratory and in clinical trials, and whether we will be able to manufacture such products at a commercially competitive price. In addition, given the very high costs of development of therapeutic products, we anticipate having to partner with larger pharmaceutical companies to bring therapeutic products to market. The terms of such partnership arrangements along with our related financial obligations cannot be determined at this time and the timing of completion of the approval of such products will likely not be within our sole control.
Marketing Expenses
Marketing expenditures were $42,545 for the quarter ended March 31, 2014, compared with $38,427 for the quarter ended March 31, 2013. The Corporation expects that marketing expenditures will increase if and when new products are launched on the market.
General and Administrative Expenses
General and administrative expenses were $1,255,563 for the quarter ended March 31, 2014, compared with $420,980 for the quarter ended March 31, 2013. The increase in general and administrative expenditures is due to stock compensation charges of $792,325 for the quarter ended March 31, 2014 compared to $21,792 in the comparative period in 2013 . The Corporation expects that general and administrative expenditures will increase as new product development leads to expanded operations.
.
Contractual Obligations
Nymox has no contractual obligations of significance other than its accounts payable, accrued liabilities and the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
Contractual Obligations |
Total |
Less than 1 year |
1-3 years |
4-5 years |
Rent for laboratory and office space |
$ |
265,346 |
|
$ |
202,295 |
|
$ |
63,051 |
|
$ |
0 |
|
Insurance premium installments |
$ |
7,279 |
|
$ |
7,279 |
|
$ |
0 |
|
$ |
0 |
|
Operating leases |
$ |
22,655 |
|
$ |
14,565 |
|
$ |
8,090 |
|
$ |
0 |
|
Convertible notes |
$ |
1,070,000 |
|
$ |
0 |
|
$ |
1,070,000 |
|
$ |
0 |
|
Interest and fees on convertible notes |
$ |
228,267 |
|
$ |
85,600 |
|
$ |
142,667 |
|
$ |
0 |
|
Total Contractual Obligations other than accounts payable and accrued liabilities |
$ |
1,593,547 |
|
$ |
309,739 |
|
$ |
1,283,808 |
|
$ |
0 |
|
The redeemable preferred shares for the Corporation’s subsidiary Serex, Inc. in the amount of $400,000 have no specific terms of repayment.
6
Off-Balance Sheet Arrangements
The Corporation has no binding commitments for the purchase of property, equipment or intellectual property. The Corporation has no commitments that are not reflected in the statement of financial position except for operating leases and insurance premium installments.
Contingent liabilities
On November 24, 2014, a shareholder of the Corporation, filed a proposed class action suit in the United States District Court, District of New Jersey, against the Corporation and the President and CEO of the Corporation. The motion was heard on January 26, 2015, and was the first procedural step before any class action could be instituted. The plaintiff seeks certification of a class action on behalf of all persons, wherever they reside, who acquired the Corporation’s common stock between January 31, 2011 and November 2, 2014. The plaintiff alleges that certain of the Corporation’s disclosures failed to disclose material adverse facts that raised serious questions as to the ability to achieve significant results for NX-1207 in Phase 3 trials in light of difficulty of enrolling candidates, obtaining objective and measured results, and the placebo effect. On March 10, 2015, we were served with a class-action lawsuit. The Corporation believes that the allegations made against it in these actions are meritless and will vigorously defend the matter, although no assurance can be given with respect to the ultimate outcome of such proceedings. No provision has been recognized in these financial statements for this matter.
Transactions with Related Parties
The Corporation had no transactions with related parties in 2015, 2014 and 2013 other than those disclosed for key management personnel in note 13 of the unaudited condensed interim Consolidated Financial Statements.
Financial Position
Liquidity and Capital Resources
As of March 31, 2015, cash and receivables including tax credits receivable totalled $707,715 compared with $1,307,501 at December 31, 2014. The decrease is mainly due to a reduction of $344,096 in tax credits receivable and a decrease of $254,108 in cash. The decrease in tax credits receivable is explained by the receipt, in the first quarter of 2015, of $349,827 (CA$ 387,350) related to the 2013 tax credits receivable. The decrease in cash is due to the payments of our expenditures. In November 2013, the Corporation signed a Common Stock Private Purchase Agreement, whereby Lorros-Greyse Investments, Ltd. (the “Purchaser”) was committed to purchase up to $15 million of the Corporation’s common shares over a twenty-four month period. The agreement became effective December 3, 2013. As at May 15, 2015, twenty-five drawings were made under the Common Stock Private Purchase Agreement, for total proceeds of $6,050,000. On December 18, 2013, 48,544 common shares were issued at a price of $6.18 per share. On January 14, 2014, 69,686 common shares were issued at a price of $5.74 per share. On February 4, 2014, 61,533 common shares were issued at a price of $5.69 per share. On February 28, 2014, 62,297 common shares were issued at a price of $5.62 per share. On March 25, 2014, 65,408 common shares were issued at a price of $5.35 per share. On April 11, 2014, 28,468 common shares were issued at a price of $5.27 per share. On April 25, 2014, 29,487 common shares were issued at a price of $5.09 per share. On May 7, 2014, 63,573 common shares were issued at a price of $4.72 per share. On May 16, 2014, 59,595 common shares were issued at a price of $5.03 per share. On May 28, 2014, 29,132 common shares were issued at a price of $5.15 per share. On June 10, 2014, 31,062 common shares were issued at a price of $4.83 per share. On June 23, 2014 , 31,302 common shares were issued at a price of $4.79 per share. On July 3, 2014, 21,501 common shares were issued at a price of $4.65 per share. On July 8, 2014, 52,312 common shares were issued at a price of $4.78 per share. On July 24, 2014, 31,672 common shares were issued at a price of $4.74 per share. On August 5, 2014, 31,179 common shares were issued at a price o f $4.81 per share. On August 8, 2014, 60,926 common shares were issued at a price of $4.92 per share. On August 27, 2014, 60,048 common shares were issued at a price of $5.00 per share. On September 9, 2014, 61,703 common shares were issued at a price of $4.86 per share. On September 15, 2014, 31,049 common shares were issued at a price of $4.83 per share. On September 30, 2014, 37,406 common shares were issued at a price of $4.01 per share. On October 9, 2014, 33,791 common shares were issued at a price of $4.44 per share. On October 24, 2014, 50,040 common shares were issued at a price of $5.00 per share. On November 12, 2014, 138,889 common shares were issued at a price of $0.72 per share. On April 28, 2015, 431,344 common shares were issued at a price of $1.39 per share. At May 15, 2015, the Corporation can require the Purchaser to purchase up to $8,950,000 of common shares over the remaining term of the Agreement subject to the conditions therein. As at the date of this MD&A, the Common Stock Private Purchase Agreement, set to expire in November 2015, has not been renewed. In prior years the Corporation typically has renewed the Common Stock Private Purchase Agreement approximately one year prior to its scheduled expiry date.
The Corporation believes its current cash balance as at March 31, 2015 and anticipated funds from product sales are not sufficient to fund substantially all of its planned business operations and research and development programs over the next year. The Corporation intends to access financing through the existing Common Stock Private Purchase Agreement and/or other sources of capital in order to fund these operations and activities over the next year. The Corporation cannot assure you that it will be able to secure additional financing on favorable terms or at all.
The top-line failure of the two Phase 3 studies of NX-1207 for BPH, announced by the Corporation on November 2, 2014, materially affects the Corporation’s current ability to fund its operations, meet its cash flow requirements, realize its assets and discharge its obligations. The Corporation’s ability to raise capital through the Common Stock Private Purchase Agreement is subject to the Corporation complying with general covenants in the Agreement in order to draw on its facility including maintaining its stock exchange listing and registration requirements and having no material adverse effects, as defined in the Agreement, with
7
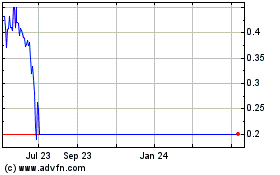
respect to the business and operations of the Corporation. On November 2, 2014, the Corporation announced that the Corporation’s Phase 3 trials of its investigational drug product, NX-1207, for the treatment of benign prostatic hyperplasia (BPH), NX02-0017 and NX02-0018, had failed to meet their primary endpoints. On November 3, 2014, the Corporation’s stock price fell from its previous close of $5.14 to a closing price of $0.93 equaling, an 82% decline. As of May 15, 2015, the Corporation has not received any communications from the Purchaser that it will not honor the Corporation’s future draw-down notices under the agreement or that it intends to terminate the agreement. On April 28, 2015, the Corporation completed a drawdown of $600,000 pursuant to the Agreement.
If the Purchaser does not purchase the Corporation`s common shares as provided for under the agreement, or if the agreement is not renewed, the Corporation will have to seek other sources of financing in order to be able to pay its obligations as they become due, which could have an impact on its ability to continue as a going concern.
The Corporation’s ability to raise capital through the Agreement and other sources of financing will be impacted by the marke t price and trading volumes of its common shares. The results of the NX02-0017 and NX02-0018 clinical trials may adversely affect the Corporation’s ability to raise capital on a timely basis, requiring the Corporation to reduce its cash requirements by elimin ating or deferring spending on research, development and corporate activities. In addition, other sources of financing may not be available or may be available only at a price or on terms that are not favorable to the Corporation.
In addition to financing operations through the issuance of equity, the Corporation may also secure additional funding through the issuance of debt, licensing or partnering products in development, increasing revenue from our products, or realizing on intellectual property and other assets. There can be no assurances that the Corporation will be successful in realizing on any such potential opportunities for additional funding at a price or on terms that are favorable to the Corporation.
On December 16, 2014, the Corporation issued secured convertible notes through a private placement for aggregate gross proceeds of $1,070,000 which bear interest at 6% per annum, payable quarterly with a maximum term of 3 years. The Corporation will also pay an administrative fee of 2% per annum on the outstanding principal amount, calculated quarterly and paid at the same time that the interest are paid on these notes. The notes are convertible by the holder at any time into common shares of the Corporation at a conversion price of $0.533 per share.
On January 23, 2015 and on March 12, 2015, the Corporation completed two $200,000 private placement financing for a total of $400,000 (see note 9(b) of the condensed interim Consolidated Financial Statements). A total of 883,058 units were issued at an average price of $0.45 per unit. Each Unit is comprised of one common share and one-half of one common share purchase warrant (each whole warrant, a “Warrant”). Each Warrant entitles the holder to acquire one common share of the Corporation a t a price per share equal to U.S. $2.00 for a period 24 months following the subscription date.
Other than the financing discussed above, the Corporation does not have arranged sources of financing.
We have incurred substantial operating losses since our inception due in large part to expenditures for our research and development activities. As at March 31, 2015, we had an accumulated deficit of $98,463,028, and we have negative cash flows from operations. Excluding the non-cash deferred revenue amount, the Corporation’s working capital deficiency is $1,269,426 at March 31, 2015. Our current level of annual expenditures exceeds the anticipated revenues from sales of goods and may not be covered by additional sources of funds.
In response to the top-line twelve month failure of the two Phase 3 trials of NX-1207 for BPH, Management has taken steps to reduce expenditures going forward in the short term by staff reductions for the U.S. BPH development program for NX-1207, deferral of management salaries, and other operational changes. Management is exploring other options, including the securing of additional sources of financing. While management believes the use of the going concern assumption is appropriate, there is no assurance the above actions will be successful. The condensed interim Consolidated Financial Statements for the quarter ended March 31, 2015, do not include any adjustments or disclosures that may be necessary should the Corporation not be able to continue as a going concern. If the going concern assumption is not appropriate for the condensed interim Consolidated Financial Statements for the quarter ended March 31, 2015, then adjustments may be necessary to the carrying value and classification of assets and liabilities and reported results of operations and such adjustments could be material.
Capital disclosures
The Corporation's objective in managing capital is to ensure a sufficient liquidity position to finance its research and development activities, general and administrative expenses, working capital and overall capital expenditures, including those associated with patents. The Corporation makes every attempt to manage its liquidity to minimize shareholder dilution when possible.
The Corporation defines capital as total equity. To fund its activities, the Corporation has followed an approach that relies almost exclusively on the issuance of common shares and, during 2010, entered into a collaboration agreement. Since inception, the Corporation has financed its liquidity needs primarily through private placements and, since 2003, through a financing agreement with an investment company that has been replaced annually by a new agreement with the same purchaser (see note 9(a) -Common Stock Private Purchase Agreement of the condensed interim Consolidated Financial Statements). The Corporation intends to access financing under this agreement when appropriate to fund its research and development activities. Since 2003 through to December 2014, Lorros-Greyse has always complied with the drawdowns made pursuant to the agreement. The Corporation must comply with general covenants in order to draw on its facility including maintaining its stock exchange listing and registration requirements and having no material adverse effects, as defined in the agreement, with respect to the business a nd operations of the Corporation. As at the date of the MD&A, the Common Stock Private Purchase Agreement, set to expire in November 2015, has not been renewed.
8
On December 16, 2014, the Corporation issued secured convertible notes through a private placement for aggregate gross proceeds of $1,070,000 which bear interest at 6% per annum, payable quarterly with a maximum term of 3 years (see note 6 of the condensed interim Consolidated Financial Statements). On January 23, 2015 and on March 12, 2015, the Corporation completed two $200,000 private placement financing for a total of $400,000 (see note 9(b) of the Condensed Interim Consolidated Financial Statements).
As part of its business plan, the Corporation anticipates the need to raise financing to pursue its planned business operations and research and development programs over the next year. The Corporation intends to access financing through the existing Common Stock Private Purchase Agreement and/or other sources of capital in order to fund these operations and activities over the next year.
If the Purchaser does not purchase the Corporation`s common shares as provided for under the existing Common Stock Private Purchase Agreement, or if the agreement is not renewed, the Corporation will have to seek other sources of financing in order to be able to pay its obligations as they become due, which could have an impact on its ability to continue as a going concern.
The Corporation’s ability to raise capital through the Agreement and other sources of financing will be impacted by the marke t price and trading volumes of its common shares. The results of the NX02-0017 and NX02-0018 clinical trials may adversely affect the Corporation’s ability to raise capital on a timely basis, requiring the Corporation to reduce its cash requirements by elimin ating or deferring spending on research, development and corporate activities. In addition, other sources of financing may not be available or may be available only at a price or on terms that are not favorable to the Corporation.
The capital management objectives remain the same as for the previous fiscal year. When possible, the Corporation tries to optimize its liquidity needs by non-dilutive sources, including sales, collaboration agreements, research tax credits and interest income. The Corporation's general policy on dividends is to retain cash to keep funds available to finance its research and development and operating expenses.
Other than the financing discussed above, the Corporation does not have arranged sources of financing.
The Corporation is not subject to any capital requirements imposed by external parties other than the Nasdaq Capital Market requirements related to the Listing Rules. On December 16, 2014, the Corporation was notified, by the Nasdaq Listing Qualifications department, that the Corporation’s Nasdaq Capital Market requirements were currently deficient for the preceding 30 consecutive business days.
However, the Listing Rules provide the Corporation a compliance period of 180 calendar days in which to regain compliance. In order to regain compliance, the Corporation must maintain a minimum market value of $35 million for a minimum of ten consecutive business days and the closing bid price of the Corporation’s common share must be at least $1 for a minimum of te n consecutive business days. Failure to meet the listing requirements may lead to delisting from the Nasdaq Capital Market in which case the Corporation will consider an alternate trading platform for its common shares. On May 4, 2015, the Corporation received notification from the Nasdaq Listing Qualifications department that it had regained compliance with the listing rules.
Outstanding Share Data
As at May 15, 2015, there were 37,186,847 common shares of Nymox issued and outstanding, as well as, 6,529,500 share options are outstanding, of which 5,057,000 are currently vested. There are 548,529 warrants outstanding. In addition, the convertible notes are convertible into 2,007,504 common shares.
Subsequent events
On April 23, 2015, at a special meeting of the shareholders of the Corporation, the shareholders approved the transfer of the Corporation’s head office and domicile from Quebec, Canada to the Bahamas.
Subsequent to the end of the quarter, the Corporation granted 1,425,000 options to executive directors and officers at an exercise price of $1.15; these options are contingent upon and cannot be exercised until the Corporation’s stock trades for five consecutive days at a price of $5.00 or greater. In addition, the Corporation amended the exercise price of all of its 5,104,500 outstanding options to $1.15.
Disclosure Controls and Procedures
Disclosure controls and procedures are designed to provide reasonable assurance that information required to be disclosed is accumulated and communicated to senior management on a timely basis so that appropriate decisions can be made regarding public disclosure. The Corporation’s Chief Executive Officer and its Chief Financial Officer are responsible for establishing and maintaining disclosure controls and procedures. They are assisted in this responsibility by the Corporation’s audit committee. Based on an evaluation of the Corporation’s disclosure controls and procedures (as defined in Rule 13a-15(e) of the Securities Exchange Act of 1934 and National Instrument 52-109), the Chief Executive Officer and Chief Financial Officer have concluded that the disclosure controls and procedures were not effective as of March 31, 2015 because of the material weakness in our internal control over financial reporting that is described in our 2014 annual filings and reproduced below in “Management’s Annual Report on Internal Control Over Financial Reporting.”
However, giving full consideration to the material weakness, the Corporation’s management has concluded that the Condensed Interim Consolidated Financial Statements as of and for the quarter ended March 31, 2015 present fairly, in all material respects, the Corporation’s financial position, results of operations and cash flows for the periods disclosed in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
9
Internal Control over Financial Reporting
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes those policies and procedures that: (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Under the supervision and with the participation of our Chief Executive Officer and our Chief Financial Officer, management conducted an evaluation of the effectiveness of our internal control over financial reporting, as of December 31, 2014, based on the framework set forth in Internal Control-Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Corporation’s annual financial statements will not be prevented or detected on a timely basis. Based on its evaluation under this framework, the Chief Executive Officer and the Chief Financial Officer concluded that our internal control over financial reporting (as defined in Rules 13a-15(f) of the Securities Exchange Act and National Instrument 52-109) was not effective as of December 31, 2014 due to the material weakness described below.
Following the announcement made on November 2, 2014 concerning the results of the two U.S. Phase 3 clinical trials, Management took steps to reduce expenditures going forward, including operational staff reductions. As a result, the Corporation did not employ a sufficient complement of finance and accounting personnel at December 31, 2014 to ensure that there was proper segregation of incompatible duties related to certain processes, primarily impacting the expenditures/disbursements processes and information technology general controls (“ITGC”), and sufficient compensating controls did not exist in these areas. Specifically, because of the limited number of qualified personnel, review controls of expenditures and disbursements were not effective to ensure that expenditures and disbursements were properly authorized and recorded in the financial information system, and certain ITGCs that potentially impact two applications used for expenditures and disbursements were not effective to monitor activities of individuals with access to modify data.
While the control deficiency identified did not result in any misstatements, a reasonable possibility exists that a material misstatement to the consolidated financial statements will not be prevented or detected on a timely basis.
Internal control over financial reporting has inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. Because of such limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
Remediation Plan for Material Weakness in Internal Control over Financial Reporting
Management believes that a lack of segregation of duties is typical of companies with limited personnel and resources. Nonetheless, in response to the material weakness identified above, the Corporation, in the immediate future, intends to develop a plan with oversight from the Audit Committee of the Board of Directors to remediate the material weakness. The Corporation does not currently intend to hire additional finance personnel or engage external experts until the size and operations warrant such additional resources.
The remediation efforts expected to be implemented include the following:
|
i) |
Evaluate staffing levels and responsibilities to enhance appropriate segregation of duties where possible amongst our personnel. |
|
ii) |
Establishing a more comprehensive review and approval process for authorizing user access to financial information systems and monitoring user access to ensure that all information technology controls designed to restrict access to applications and data are operating in a manner that provides the Corporation with assurance that such access is properly restricted to the appropriate personnel. |
Changes in Internal Controls Over Financial Reporting
There have been no changes since December 31, 2014 in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
10
Changes in accounting policies:
New accounting standards and interpretations:
Issued but not yet adopted:
A number of new standards, interpretations and amendments to existing standards were issued by the IASB or International Financial Reporting Standards Interpretations Committee (“IFRS IC”). They are mandatory but not yet effective for the period ended March 31, 2015, and have not been applied in preparing these consolidated financial statements. Many of these are not applicable or are inconsequential to the Corporation and have been excluded from the discussion below.
The following standards and interpretations have been issued by the IASB and the IFRS IC and the Corporation is currently assessing their impact on the financial statements:
(a) IFRS 9, Financial Instruments:
IFRS 9 - Financial Instruments (“IFRS 9”) ultimately replaces IAS 39 – Financial Instruments: Recognition and Measurement (“IAS 39”). The replacement of IAS 39 is a three-phase project with the objective of improving and simplifying the reporting for financial instruments.
IFRS 9 (2009) introduces new requirements for the classification and measurement of financial assets. Under IFRS 9 (2009), financial assets are classified and measured based on the business model in which they are held and the characteristics of th eir contractual cash flows.
IFRS 9 (2010) introduces additional changes relating to financial liabilities.
IFRS 9 (2013) includes a new general hedge accounting standard which will align hedge accounting more closely with risk management. Special transitional requirements have been set for the application of the new general hedging model. The Corporation currently does not hedge and therefore does not anticipate this phase will have an impact on the Corporation.
This standard is effective for annual periods beginning on or after January 1, 2018 with earlier adoption permitted. The Corporation has not yet assessed the impact of the adoption of this standard on its consolidated financial statements.
(b) IFRS 15, Revenue from Contracts with Customers:
In May 2014, the IASB issued IFRS 15, Revenue from Contracts with Customers, which establishes principles for reporting the nature, amount, timing and uncertainty of revenue and cash flows arising from an entity’s contracts with customers. It provid es a single model in order to depict the transfer of promised goods or services to customers.
IFRS 15 supersedes the following standards: IAS 11, Construction Contracts, IAS 18, Revenue, IFRIC 13, Customer Loyalty Programmes, IFRIC 15, Agreements for the Construction of Real Estate, IFRIC 18, Transfers of Assets from Customers, and SIC -31, Revenue - Barter Transactions Involving Advertising Service.
The core principle of IFRS 15 is that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods and services.
IFRS 15 also includes a cohesive set of disclosure requirements that would result in an entity providing comprehensive inform ation about the nature, amount, timing and uncertainty of revenue and cash flows arising from the entity’s contracts with customers.
This standard is effective for annual periods beginning on or after January 1, 2017 with earlier adoption permitted. The Corporation has not yet assessed the impact of the adoption of this standard on its consolidated financial statements.
Forward Looking Statements
Certain statements included in this MD&A may constitute “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 and Canadian securities legislation and regulations, and are subject to important risks, uncertainties and assumptions. This forward-looking information includes amongst others, information with respect to our objectives and the strategies to achieve these objectives, as well as information with respect to our beliefs, plans, expectations, anticipations, estimates and intentions. Forward-looking statements generally can be identified by the use of forward-looking terminology such as “may”, “will”, “expect”, “intend”, “estimate”, “anticipate”, “plan”, “foresee”, “believe” or “continue” or the negatives of these terms or variations of them or similar terminology. We refer you to the Corporation’s filings with the Can adian securities regulatory authorities and the U.S. Securities and Exchange Commission, as well as the “Risk Factors” section of this MD&A, and of our Form 20-F, for a discussion of the various factors that may affect the Corporation’s future results. The results or events predicted in such forward-looking information may differ materially from actual results or events.
Factors that could cause actual results or plans to differ materially from those projected in forward -looking statements made by, or on behalf of, the Corporation, many of which are beyond our control, include the Corporation’s ability to:
-
identify and capitalize on possible collaboration, strategic partnering or divestiture opportunities;
-
obtain suitable financing to support its operations and clinical trials;
-
access financing under the Common Stock Private Purchase Agreement;
-
successfully defend pending and/or unforeseeable future litigation;
11
-
manage its growth and the commercialization of its products;
-
achieve operating efficiencies as it progresses from a development-stage to a later-stage biotechnology corporation;
-
successfully compete in its markets;
-
realize the results it anticipates from the clinical trials of its products;
-
overcome recent negative results from its clinical trials;
-
succeed in finding and retaining joint venture and collaboration partners to assist it in the successful marketing, distribution and commercialization of its products;
-
achieve regulatory clearances for its products;
-
obtain on commercially reasonable terms adequate product liability insurance for its commercialized products and avoid product liability claims;
-
adequately protect its proprietary information and technology from competitors and avoid infringement of proprietary information and technology of its competitors;
-
assure that its products, if successfully developed and commercialized following regulatory approval, are not rendered obsolete by products or technologies of competitors; and
-
not encounter problems with third parties, including key personnel, upon whom it is dependent.
Forward-looking statements do not take into account the effect that transactions or non-recurring or other special items announced or occurring after the statements are made have on the Corporation’s business. For example, they do not include the effect of business dispositions, acquisitions, other business transactions, asset write downs or other charges announced or occurring after forward-looking statements are made. The financial impact of such transactions and non-recurring and other special items can be complex and necessarily depends on the facts particular to each of them.
We believe that the expectations represented by our forward-looking statements are reasonable, yet there can be no assurance that such expectations will prove to be correct. Furthermore, the forward-looking statements contained in this report are made as of the date of this report, and we do not undertake any obligation to update publicly or to revise any of the included forward -looking statements, whether as a result of new information, future events or otherwise unless required by applicable legislation or regulation. The forward-looking statements contained in this report are expressly qualified by this cautionary statement.
12
Condensed Interim Consolidated Financial Statements of
(Unaudited)
NYMOX PHARMACEUTICAL
CORPORATION
Three-month periods ended March 31, 2015, 2014 and 2013
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Condensed Interim Consolidated Financial Statements |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
Financial Statements
|
|
|
|
Condensed Interim Consolidated Statements of Financial Position |
1 |
|
Condensed Interim Consolidated Statements of Operations and Comprehensive Income (Loss) |
2 |
|
Condensed Interim Consolidated Statements of Changes in Equity |
3 |
|
Condensed Interim Consolidated Statements of Cash Flows |
5 |
Notes to Condensed Interim Consolidated Financial Statements
|
|
|
| 1. |
Business activities and future operations |
6 |
| 2. |
Basis of preparation |
7 |
| 3. |
Significant accounting policies |
8 |
| 4. |
Accounts payable and accrued liabilities |
8 |
| 5. |
Other liabilities |
9 |
| 6. |
Convertible notes |
9 |
| 7. |
Licensing revenues and deferred revenue |
11 |
| 8. |
Preferred shares of a subsidiary and non-controlling interest |
11 |
| 9. |
Share capital |
12 |
| 10. |
Contingencies |
16 |
| 11. |
Income taxes |
16 |
| 12. |
Earnings per share |
17 |
| 13. |
Related parties |
17 |
| 14. |
Subsequent event |
17 |
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Condensed Interim Consolidated Statements of Financial Position |
| (Unaudited) |
| |
| March 31, 2015 and December 31, 2014 |
| (in US dollars) |
|
|
|
|
|
|
|
|
| |
Note |
|
March 31, |
|
|
December 31, |
|
| |
|
|
2015 |
|
|
2014 |
|
| |
|
|
|
|
|
|
|
| Assets |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| Current assets: |
|
|
|
|
|
|
|
Cash |
|
$ |
378,164 |
|
$ |
632,272 |
|
Accounts receivable |
|
|
24,030 |
|
|
12,959 |
|
Other receivables |
|
|
34,963 |
|
|
47,616 |
|
Research tax credits receivable |
|
|
270,558 |
|
|
614,654 |
|
Prepaid expenses |
|
|
30,000 |
|
|
– |
|
Inventories |
|
|
73,365 |
|
|
88,269 |
|
Total current assets |
|
|
811,080 |
|
|
1,395,770 |
|
| |
|
|
|
|
|
|
|
| Non-current assets: |
|
|
|
|
|
|
|
Security deposit |
|
|
17,396 |
|
|
17,396 |
|
Property and equipment |
|
|
7,629 |
|
|
9,400 |
|
Total non-current assets |
|
|
25,025 |
|
|
26,796 |
|
| |
|
|
|
|
|
|
|
| Total assets |
|
$ |
836,105 |
|
$ |
1,422,566 |
|
| |
|
|
|
|
|
|
|
| Liabilities and Equity |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
| Current liabilities: |
|
|
|
|
|
|
|
Accounts payable and accrued liabilities |
4 |
$ |
2,080,506 |
|
$ |
1,976,145 |
|
Deferred revenue |
7 |
|
– |
|
|
2,508,533 |
|
Total current liabilities |
|
|
2,080,506 |
|
|
4,484,678 |
|
| |
|
|
|
|
|
|
|
| Non-current liabilities : |
|
|
|
|
|
|
|
Convertible notes |
6 |
|
743,440 |
|
|
718,831 |
|
Preferred shares of a subsidiary |
8 |
|
400,000 |
|
|
400,000 |
|
Total non-current liabilities |
|
|
1,143,440 |
|
|
1,118,831 |
|
| |
|
|
|
|
|
|
|
| Equity: |
|
|
|
|
|
|
|
Share capital |
9 |
|
81,568,578 |
|
|
81,227,058 |
|
Share capital subscription |
|
|
– |
|
|
200,000 |
|
Warrants |
|
|
88,012 |
|
|
29,532 |
|
Equity component of convertible notes |
6 |
|
188,937 |
|
|
188,937 |
|
Additional paid-in capital |
|
|
13,829,660 |
|
|
13,813,109 |
|
Deficit |
|
|
(98,463,028) |
|
|
(100,039,579) |
|
Total equity attributable to the equity holders of the Corporation |
|
|
(2,787,841) |
|
|
(4,580,943) |
|
| |
|
|
|
|
|
|
|
| Non-controlling interest |
8 |
|
400,000 |
|
|
400,000 |
|
| Total equity (deficiency) |
|
|
(2,387,841) |
|
|
(4,180,943) |
|
| |
|
|
|
|
|
|
|
| Business activities and future operations |
1 |
|
|
|
|
|
|
| Contingencies |
10 |
|
|
|
|
|
|
| Subsequent event |
14 |
|
|
|
|
|
|
| Total liabilities and equity |
|
$ |
836,105 |
|
$ |
1,422,566 |
|
See accompanying notes to the condensed interim consolidated financial statements.
1
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Condensed Interim Consolidated Statements of Operations and Comprehensive Income (Loss) |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| (in US dollars) |
|
|
|
|
|
|
|
|
|
|
|
| |
Note |
|
2015 |
|
|
2014 |
|
|
2013 |
|
| |
|
|
|
|
|
|
|
|
|
|
| Revenues: |
|
|
|
|
|
|
|
|
|
|
Sales of goods |
|
$ |
75,387 |
|
$ |
78,164 |
|
$ |
184,246 |
|
Licensing revenues: |
|
|
|
|
|
|
|
|
|
|
Upfront payment |
7 |
|
2,508,533 |
|
|
654,400 |
|
|
654,400 |
|
| |
|
|
2,583,920 |
|
|
732,564 |
|
|
838,646 |
|
| |
|
|
|
|
|
|
|
|
|
|
| Expenses: |
|
|
|
|
|
|
|
|
|
|
Research and development |
9 (c) |
|
559,303 |
|
|
2,061,038 |
|
|
1,471,153 |
|
Less research tax credits |
|
|
– |
|
|
(96,412) |
|
|
(64,377) |
|
Net research and development |
|
|
559,303 |
|
|
1,964,626 |
|
|
1,406,776 |
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
9 (c) |
|
348,779 |
|
|
1,255,563 |
|
|
420,980 |
|
Marketing |
9 (c) |
|
9,319 |
|
|
42,545 |
|
|
38,427 |
|
Cost of sales |
|
|
37,481 |
|
|
57,040 |
|
|
66,988 |
|
Total expenses |
|
|
954,882 |
|
|
3,319,774 |
|
|
1,933,171 |
|
| |
|
|
|
|
|
|
|
|
|
| Net income (loss) from operating activities |
|
1,629,038 |
|
|
(2,587,210) |
|
|
(1,094,525) |
|
| |
|
|
|
|
|
|
|
|
|
|
| Net finance costs |
|
|
(52,487) |
|
|
(5,606) |
|
|
619 |
|
Net income (loss) and comprehensive income (loss) attributable to the equity holders of the Corporation |
|
$ |
1,576,551 |
|
$ |
(2,592,816) |
|
$ |
(1,093,906) |
|
| |
|
|
|
|
|
|
|
|
|
|
| Net income (loss) per share |
|
|
|
|
|
|
|
|
|
|
Basic |
12 |
$ |
0.04 |
|
$ |
(0.07) |
|
$ |
(0.03) |
|
Diluted |
12 |
|
0.04 |
|
|
(0.07) |
|
|
(0.03) |
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding |
|
|
|
|
|
|
|
|
|
|
Basic |
12 |
|
36,272,978 |
|
|
34,797,302 |
|
|
33,679,486 |
|
Diluted |
12 |
|
38,280,483 |
|
|
34,797,302 |
|
|
33,679,486 |
|
See accompanying notes to the condensed interim consolidated financial statements.
2
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Condensed Interim Consolidated Statements of Changes in Equity |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| (in US dollars) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Attributable to equity holders of the Corporation |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
component |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
of |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Share capital |
|
|
Share capital |
|
|
|
|
|
paid-in |
|
|
convertible |
|
|
|
|
|
|
Non-controlling |
|
|
Total |
|
| |
Note |
|
Number |
|
|
Dollars |
|
|
subscription |
|
|
Warrants |
|
|
capital |
|
|
notes |
|
|
Deficit |
|
|
Total |
|
|
interest |
|
|
equity |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2014 |
|
|
35,872,445 |
|
$ |
81,227,058 |
|
$ |
200,000 |
|
$ |
29,532 |
|
$ |
13,813,109 |
|
$ |
188,937 |
|
$ |
(100,039,579) |
|
$ |
(4,580,943) |
|
$ |
400,000 |
|
$ |
(4,180,943) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transactions with owners, recorded directly in equity: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of units |
9(a) |
|
883,058 |
|
|
341,520 |
|
|
(200,000) |
|
|
58,480 |
|
|
– |
|
|
– |
|
|
– |
|
|
200,000 |
|
|
– |
|
|
200,000 |
|
Stock-based compensation |
9(c) |
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
16,551 |
|
|
– |
|
|
– |
|
|
16,551 |
|
|
– |
|
|
16,551 |
|
| Total contributions by owners |
|
|
883,058 |
|
|
341,520 |
|
|
(200,000) |
|
|
58,480 |
|
|
16,551 |
|
|
– |
|
|
– |
|
|
216,551 |
|
|
– |
|
|
216,551 |
|
Net income and comprehensive income |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
1,576,551 |
|
|
1,576,551 |
|
|
– |
|
|
1,576,551 |
|
| Balance, March 31, 2015 |
|
|
36,755,503 |
|
$ |
81,568,578 |
|
$ |
– |
|
$ |
88,012 |
|
$ |
13,829,660 |
|
$ |
188,937 |
|
$ |
(98,463,028) |
|
$ |
(2,787,841) |
|
$ |
400,000 |
|
$ |
(2,387,841) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2013 |
|
|
34,672,157 |
|
$ |
76,046,549 |
|
$ |
– |
|
$ |
– |
|
$ |
12,631,067 |
|
$ |
– |
|
$ |
(95,135,986) |
|
$ |
(6,458,370) |
|
$ |
400,000 |
|
$ |
(6,058,370) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transactions with owners, recorded directly in equity: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of share capital |
9(a) |
|
258,924 |
|
|
1,450,000 |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
1,450,000 |
|
|
– |
|
|
1,450,000 |
|
Share issue costs |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
(72,500) |
|
|
(72,500) |
|
|
– |
|
|
(72,500) |
|
Options settled |
5 |
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
(397,872) |
|
|
– |
|
|
– |
|
|
(397,872) |
|
|
– |
|
|
(397,872) |
|
Stock-based compensation |
9(c) |
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
1,420,185 |
|
|
– |
|
|
– |
|
|
1,420,185 |
|
|
– |
|
|
1,420,185 |
|
| Total contributions by owners |
|
|
258,924 |
|
|
1,450,000 |
|
|
– |
|
|
– |
|
|
1,022,313 |
|
|
– |
|
|
(72,500) |
|
|
2,399,813 |
|
|
– |
|
|
2,399,813 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss and comprehensive loss |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
(2,592,816) |
|
|
(2,592,816) |
|
|
– |
|
|
(2,592,816) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, March 31, 2014 |
|
|
34,931,081 |
|
$ |
77,496,549 |
|
$ |
– |
|
$ |
– |
|
$ |
13,653,380 |
|
$ |
– |
|
$ |
(97,801,302) |
|
$ |
(6,651,373) |
|
$ |
400,000 |
|
$ |
(6,251,373) |
|
See accompanying notes to the condensed interim consolidated financial statements.
3
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Condensed Interim Consolidated Statements of Changes in Equity, Continued |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| (in US dollars) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Attributable to equity holders of the Corporation |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
component |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
of |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Share capital |
|
|
Share capital |
|
|
|
|
|
paid-in |
|
|
convertible |
|
|
|
|
|
|
Non-controlling |
|
|
Total |
|
| |
Note |
|
Number |
|
|
Dollars |
|
|
subscription |
|
|
Warrants |
|
|
capital |
|
|
debt |
|
|
Deficit |
|
|
Total |
|
|
interest |
|
|
equity |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, December 31, 2012 |
|
|
33,572,442 |
|
$ |
69,705,389 |
|
$ |
– |
|
$ |
– |
|
$ |
12,362,281 |
|
$ |
– |
|
$ |
(89,912,383) |
|
$ |
(7,844,713) |
|
$ |
400,000 |
|
$ |
(7,444,713) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transactions with owners, recorded directly in equity: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of share capital |
9(a) |
|
368,035 |
|
|
2,100,000 |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
2,100,000 |
|
|
– |
|
|
2,100,000 |
|
Share issue costs |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
(105,000) |
|
|
(105,000) |
|
|
– |
|
|
(105,000) |
|
Exercise of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash |
9(b) |
|
5,440 |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
Ascribed value |
|
|
– |
|
|
11,020 |
|
|
– |
|
|
– |
|
|
(11,020) |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
Stock-based compensation |
9(c) |
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
26,640 |
|
|
– |
|
|
– |
|
|
26,640 |
|
|
– |
|
|
26,640 |
|
| Total contributions by owners |
|
|
373,475 |
|
|
2,111,020 |
|
|
– |
|
|
– |
|
|
15,620 |
|
|
– |
|
|
(105,000) |
|
|
2,021,640 |
|
|
– |
|
|
2,021,640 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Net loss and comprehensive loss |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
– |
|
|
(1,093,906) |
|
|
(1,093,906) |
|
|
– |
|
|
(1,093,906) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Balance, March 31, 2013 |
|
|
33,945,917 |
|
$ |
71,816,409 |
|
$ |
– |
|
$ |
– |
|
$ |
12,377,901 |
|
$ |
– |
|
$ |
(91,111,289) |
|
$ |
(6,916,979) |
|
$ |
400,000 |
|
$ |
(6,516,979) |
|
See accompanying notes to the condensed interim consolidated financial statements.
4
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Consolidated Statements of Cash Flows |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| (in US dollars) |
|
|
|
|
|
|
|
|
|
|
|
| |
Note |
|
2015 |
|
|
2014 |
|
|
2013 |
|
| |
|
|
|
|
|
|
|
|
|
|
| Cash flows from (used in) operating activities: |
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
|
$ |
1,576,551 |
|
$ |
(2,592,816) |
|
$ |
(1,093,906) |
|
Adjustments for: |
|
|
|
|
|
|
|
|
|
|
Depreciation of property and equipment |
|
|
1,771 |
|
|
1,540 |
|
|
2,153 |
|
Stock-based compensation |
|
|
16,551 |
|
|
1,420,185 |
|
|
26,640 |
|
Accretion expense |
|
|
24,609 |
|
|
8,146 |
|
|
- |
|
Changes in non-cash operating balances: |
|
|
|
|
|
|
|
|
|
|
Accounts receivable and other receivables |
|
|
1,582 |
|
|
168,252 |
|
|
(73,619) |
|
Research tax credits receivable |
|
|
344,096 |
|
|
(96,412) |
|
|
(64,377) |
|
Prepaid expenses |
|
|
(30,000) |
|
|
(38,069) |
|
|
29,237 |
|
Inventories |
|
|
14,904 |
|
|
7,963 |
|
|
(73,452) |
|
Accounts payable and accrued liabilities |
|
|
147,461 |
|
|
384,150 |
|
|
253,522 |
|
Deferred revenue |
|
|
(2,508,533) |
|
|
(654,400) |
|
|
(654,400) |
|
| |
|
|
(411,008) |
|
|
(1,391,461) |
|
|
(1,648,202) |
|
| Cash flows from (used in) financing activities: |
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of share capital |
|
|
- |
|
|
1,450,000 |
|
|
2,100,000 |
|
Proceeds from issuance of units |
|
|
200,000 |
|
|
- |
|
|
- |
|
Share issue costs |
|
|
- |
|
|
(72,500) |
|
|
(105,000) |
|
Payments under option settlement agreements |
|
|
(43,100) |
|
|
(30,199) |
|
|
- |
|
| |
|
|
156,900 |
|
|
1,347,301 |
|
|
1,995,000 |
|
| Cash flows used in investing activities: |
|
|
|
|
|
|
|
|
|
|
Additions to property and equipment |
|
|
- |
|
|
(2,058) |
|
|
(1,440) |
|
| Net (decrease) increase in cash |
|
|
(254,108) |
|
|
(46,218) |
|
|
345,358 |
|
| Cash, beginning of the period |
|
|
632,272 |
|
|
295,523 |
|
|
1,030,075 |
|
| Cash, end of the period |
|
$ |
378,164 |
|
$ |
249,305 |
|
$ |
1,375,433 |
|
See accompanying notes to the condensed interim consolidated financial statements
5
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 1. |
Business activities and future operations: |
Nymox Pharmaceutical Corporation is a company domiciled in Canada and is incorporated under the Canada Business Corporations Act. On April 23, 2015, the shareholders of the Corporation, approved the transfer of the Corporation’s head office and domicile from Quebec, Canada to the Bahamas (see note 14). Nymox Pharmaceutical Corporation including its subsidiaries, Nymox Corporation, a Delaware Corporation, and Serex Inc. of New Jersey (together referred to as the “Corporation”), is a biopharmaceutical corporation, which specializes in the research and development of products for the aging population. The head office of the Corporation is located at 9900 Cavendish Boulevard, Saint-Laurent, Québec. The Corporation currently markets NicAlertTM and TobacAlertTM, tests that use urine or saliva to detect use of tobacco products. Since 1989, the Corporation’s activities and resources have been primarily focused on developing certain pharmaceutical technologies. Since 2002, the Corporation has been developing its novel proprietary drug candidate, NX-1207, for the treatment of benign prostatic hyperplasia (BPH) and, since 2012, for the treatment of low-grade localized prostate cancer. The Corporation also has an extensive patent portfolio covering its marketed products, its investigational drug as well as other therapeutic and diagnostic indications.
The Corporation is subject to a number of risks, including the successful development and marketing of its technologies and maintaining access to existing financing arrangements under the Common Stock Private Purchase Agreement (the “Agreement”) referred to in note 9 (a). The Corporation depends on this financing, private placements and other types of financing as well as collaboration agreements, to fund its operations. In order to achieve its business plan and the realization of its assets and liabilities in the normal course of operations, the Corporation anticipates the need to raise additional capital and/or achieve sales and other revenue-generating activities.
The Corporation is listed on the Nasdaq Stock Market. On December 16, 2014, the Corporation was notified by the Nasdaq Listing Qualifications department that the Corporation’s Nasdaq Capital Market requirements were currently deficient for the preceding 30 consecutive business days. However, the Listing Rules provide the Corporation a compliance period of 180 calendar days in which to regain compliance. In order to do so, the Corporation must maintain a minimum market value of $35 million for a minimum of ten consecutive business days and the closing bid price of the Corporation’s common share must be at least $1 for a minimum of ten consecutive business days. Failure to meet the listing requirements may lead to delisting from the Nasdaq Capital Market in which case the Corporation will consider an alternate trading platform for its common shares. On May 4, 2015, the Corporation received notification from the Nasdaq Listing Qualifications department that it had regained compliance with the listing rules.
On November 2, 2014, the Corporation issued a press release announced that the top-line results of the Phase 3 NX02-0017 and NX02-0018 U.S. clinical trials of NX-1207 for BPH at 12 months post-treatment were not statistically significant compared to placebo. The Corporation is in the process of further data analysis and assessments of the two studies, and expects to continue its efforts to work on the development program. Management has taken steps to reduce expenditures going forward in the short term by staff reductions, deferral of management salaries,
6
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 1. |
Business activities and future operations (continued): |
and operational changes. After the top-line statistical failure of Corporation's U.S. Phase 3 studies NX02-0017 and NX02-0018 at 12 months post-treatment, Recordati, in view of these results, announced, on February 12, 2015, its decision to prematurely interrupt the European clinical trial (see note 7). The NX-1207 program for low grade localized prostate cancer continues. The Corporation announced positive top line results for its Phase 2 study of NX-1207 for localized prostate cancer in April 2014. The Corporation is in the process of working towards definitive studies for this indication.
The failure of the two Phase 3 studies of NX-1207 for BPH materially affects the Corporation’s current ability to fund its operations, meet its cash flow requirements, realize its assets and discharge its obligations. Under the Common Stock Private Purchase Agreement, the Corporation must adhere to general covenants in order to draw on its facility, including maintaining its stock exchange listing and registration requirements and having no material adverse effects, as defined in the Agreement, with respect to the business and operations of the Corporation. In the past, the Corporation has been successful in obtaining the needed financing pursuant to the agreement. As of the date of the financial statements, the Corporation has not received any communication from the purchaser that it will not honor the Corporation`s future draw-down notices under the Agreement or that it intends to terminate the Agreement. As of the date of the financial statements, the Common Stock Private Purchase Agreement, set to expire in November 2015, has not been renewed. Subsequent to March 31, 2015, the Corporation completed a drawdown of $600,000 pursuant to this Agreement (see note 9(a)).
Management believes that current cash balances as at March 31, 2015 and anticipated funds from product sales are not sufficient to fund substantially all of its planned business operations and research and development programs over the next year. The Corporation intends to access financing through the existing Common Stock Private Purchase Agreement and/or other sources of capital in order to fund these operations and activities over the next year.
If the purchaser does not purchase the Corporation`s common shares as provided for under the Agreement, or if the Agreement is not renewed, the Corporation will have to seek other sources of financing in order to be able to pay its obligations as they become due, which could have an impact on its ability to continue as a going concern. Considering recent developments and the need for additional financing, there exists a material uncertainty that casts substantial doubt about the Corporation’s ability to continue as a going concern. These financial statements do not reflect adjustments that would be necessary if the going concern assumption was not appropriate. If the going concern assumption is not appropriate, then adjustments may be necessary to the carrying value and classification of assets and liabilities and reported results of operations and such adjustments could be material.
|
(a) |
Statement of compliance: |
The condensed interim consolidated financial statements of the Corporation have been prepared in accordance with International Financial Reporting Standards (“IFRS”) and its
7
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 2. |
Basis of preparation (continued): |
|
|
|
(a) |
Statement of compliance (continued): |
interpretations as issued by the International Accounting Standards Board (“IASB”) and in accordance with IAS 34, Interim Financial Reporting. The condensed interim consolidated financial statements do not include all of the information required for full annual financial statements and accordingly should be read in connection with the previously issued annual financial statements of the Corporation as at and for the year ended December 31, 2014.
The condensed interim consolidated financial statements were authorized for issuance by the Board of Directors on May 15, 2015.
|
(b) |
Basis of measurement: |
The condensed interim consolidated financial statements have been prepared on a going concern and on the historical cost basis. The functional and presentation currency of the Corporation is the US dollar.
| 3. |
Significant accounting policies: |
The accounting policies described in the Corporation’s 2014 annual consolidated financial statements have been applied consistently to all periods presented in these condensed interim consolidated financial statements.
Accounting estimates and judgments:
The preparation of the condensed interim consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of accounting policies and reported amounts of assets and liabilities, income and expense. Actual results may differ from those estimates.
In preparing these condensed interim consolidated financial statements, the significant judgments made by management in applying the Corporation’s significant accounting policies and key sources of information were the same as those applied to the consolidated financial statements for the year ended December 31, 2014.
| 4. |
Accounts payable and accrued liabilities: |
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
|
2015 |
|
|
2014 |
|
|
|
|
|
|
|
|
|
|
Accounts payable |
$ |
1,748,410 |
|
$ |
1,484,335 |
|
|
Accrued liabilities: |
|
|
|
|
|
|
|
Payroll related liabilities |
|
130,340 |
|
|
69,447 |
|
|
Other accrued liabilities |
|
201,756 |
|
|
379,263 |
|
|
Other liabilities (note 5) |
|
- |
|
|
43,100 |
|
|
Total accounts payable and accrued liabilities |
$ |
2,080,506 |
|
$ |
1,976,145 |
|
8
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
On January 22, 2014, in connection with the departure of the former Chief Financial Officer, the Corporation entered into an agreement with him, whereby he was entitled to receive CA$500,000 payable in equal bi-monthly installments until July 26, 2016, in exchange for cancelling all of his outstanding 240,000 stock options. This exchange of options for future cash payments was accounted for as an equity transaction and therefore an accrued liability and a reduction to additional paid in capital of $397,872 (CA$441,589), the discounted value of the total consideration, was recorded during the first quarter of 2014. All future payments reduced the accrued liability balance, net of the related accretion expense.
On December 15, 2014, the Corporation and the former Chief Financial Officer entered into an agreement to amend the term and the amounts payable under the initial agreement. Under the amended agreement, the Corporation paid a total of $85,187 (CA$100,000), $42,996 (CA$50,000) of which was paid in December 2014, and the remaining $42,191 (CA$50,000) was paid in January 2015. There will be no further payments or obligations of any amounts from the Corporation.
At the time of the amended agreement, the accrued liability was $280,136 (CA$ 320,475). As a result, the difference between the carrying amount of the accrued current and non-current liability and the amended settlement amount was recognized as a gain in the amount of $189,575 (CA$220,475) on the consolidated statements of operations and comprehensive loss as at December 2014.
As at March 31, 2015, no further amounts (December 31, 2014, $43,100 (CA$50,000)), remain to be paid under this agreement.
The carrying value of the convertible notes consist of the following:
|
|
|
|
|
|
|
|
March 31, |
|
|
|
|
2015 |
|
|
|
|
|
|
|
Balance, beginning of the period |
|
718,831 |
|
|
Accretion expense |
|
24,609 |
|
|
Balance, end of the period |
|
743,440 |
|
On December 16, 2014, the Corporation issued secured convertible notes through a private placement for aggregate gross proceeds of $1,070,000, which bear interest at 6% per annum, payable quarterly with a maximum term of 3 years. The Corporation will also pay an administrative fee of 2% per annum on the outstanding principal amount, calculated quarterly and paid at the same time that interest is paid on these notes. The Corporation has agreed to grant a first lien on its assets to secure its obligations under the note. The notes are convertible at the holder’s option at any time into common shares of the Corporation at a conversion price of $0.533 per share.
9
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 6. |
Convertible notes (continued): |
The convertible note has been classified as a liability at its estimated fair value with the residual allocated to the conversion feature. As a result, the recorded liability for the convertible note is lower than its face value which is characterized as a debt discount. The conversion feature is classified as equity. The Corporation fair valued the debt component using a discounted cash flow model.
As of December 16, 2014, the closing date of the convertible note, the value of the debt component and the conversion option were as follows:
|
|
|
|
|
|
Balance at December 31, 2013 |
$ |
- |
|
|
Convertible notes issued for cash |
|
1,070,000 |
|
|
Issuance costs paid in cash |
|
(125,700) |
|
|
Issuance costs paid in shares |
|
(7,000) |
|
|
Issuance costs paid through issuance of warrants |
|
(29,532) |
|
|
Total amount to be attributed at date of issuance |
$ |
907,768 |
|
|
|
|
|
|
|
Debt component |
$ |
718,831 |
|
|
Equity component |
|
188,937 |
|
|
|
$ |
907,768 |
|
In connection with the issuance of the convertible notes, the Corporation issued 107,000 warrants to the placement agent as part of the placement fee. The warrants are classified as equity as they meet the criteria for such classification. See note 9(e).
Using the effective interest rate method and the 23.57% rate implicit in the calculation, the difference of $351,169 between the amount attributed to the debt component and the face value of the convertible note, characterized as the debt discount, will be accreted to the fair value as a finance cost over the term of the convertible notes.
Any time after December 16, 2014, the Corporation may, with 30 days written notice, prepay the amounts due without premium or penalty including all outstanding interest accumulated to the date of prepayment, when the following conditions are met: if the per share closing sale price is at least 200% of the conversion price for twenty (20) consecutive trading days prior to the date of the prepayment notice, and the average daily volume of the Corporation’s common stock for the fifty (50) trading days prior to the date of the prepayment notice is a minimum of 100,000 shares per day. In addition, the Corporation may only prepay the amounts due if the Corporation has filed a registration statement with the Securities and Exchange Commission and such registration statement is then effective for the registration of all shares into which the notes may be converted.
10
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 7. |
Licensing revenues and deferred revenue: |
On December 16, 2010, the Corporation signed a license and collaboration agreement with Recordati Ireland Ltd. (“Recordati”), a European pharmaceutical group, for the development and commercialization of NX-1207 in Europe, including Russia and the CIS, the Middle East, the Maghreb area of North Africa and South Africa. The license and collaboration agreement covers the use of NX-1207 for the treatment of benign prostatic hyperplasia (“BPH”) as the initial indication for development and commercialization. Recordati made an upfront payment to the Corporation of €10,000,000 (US$13,088,000), in December 2010. The agreement provides for further payments to be made upon regulatory approval, sales milestones payments, and tiered supply and royalty payments of a minimum of 26% to increase progressively up to 40% of total net sales in the case specific contractual conditions are achieved.
The upfront payment of $13,088,000 has been deferred and was being recognized as revenue on a systematic basis over the estimated service period of five years, of which approximately one year remained at December 31, 2014. In the first quarter of 2015, this estimated service period was modified based on additional information received by the Corporation. On February 12, 2015, Recordati S.p.A. announced their decision to prematurely interrupt the European clinical trial before having reached the expected target of 340 patients. As at this date, the Corporation determined that the estimated service period for recognizing the upfront payment received in December 2010 concluded effective February 12, 2015. Consequently, the Corporation recognized, as revenue, the amount of $2,508,533 which represented the remaining deferred revenue relating to the upfront payment received from Recordati in December 2010. In the three-month period ended March 31, 2015, an amount of $2,508,533 (2014 - $654,400; 2013 -$654,400) was recognized as revenue related to this upfront payment. As at March 31, 2015, the deferred revenue related to this transaction amounted to nil (as at December 31, 2014 -$2,508,533).
| 8. |
Preferred shares of a subsidiary and non-controlling interest: |
The preferred shares of a subsidiary and the non-controlling interest relate to redeemable and/or convertible preferred shares of Serex Inc. in the amount of $800,000. These preferred shares are convertible into common shares of Serex Inc. at a price of $3.946 per share. Up to 50% of the preferred shares are redeemable at any time at the option of the preferred shareholders for their issue price, subject to holders with at least 51% of the face value of the preferred shares asking for redemption, and sufficient funds being available in Serex Inc. These redeemable preferred shares in the amount of $400,000 have been presented as a liability in the statements of financial position and are measured at their issue price which is also the redemption value. The non-redeemable portion is presented within equity, separately from equity of the owners of the Corporation, as non-controlling interest.
11
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
|
|
|
2015 |
|
|
2014 |
|
|
|
|
|
|
|
|
|
|
Authorized: |
|
|
|
|
|
|
|
An unlimited number of common shares, |
|
|
|
|
|
|
|
at no par value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued, outstanding and fully paid: |
|
|
|
|
|
|
|
Number of common shares |
|
36,755,503 |
|
|
35,872,445 |
|
|
Dollars |
$ |
81,568,578 |
|
$ |
81,227,058 |
|
|
|
|
|
|
|
|
|
The holders of common shares are entitled to receive dividends as declared, which is at the discretion of the Corporation, and are entitled to one vote per share at the annual general meeting of the Corporation. The Corporation has never paid any dividends.
|
(a) |
Common Stock Private Purchase Agreement: |
In November 2013, the Corporation entered into a Common Stock Private Purchase Agreement with an investment company (the “Purchaser”) that established the terms and conditions for the purchase of common shares by the Purchaser. In general, the Corporation can, at its discretion, require the Purchaser to purchase up to $15 million of common shares over a 24-month period based on notices given by the Corporation. The Corporation must comply with general covenants in order to draw on its facility, including maintaining its stock exchange listing and registration requirements and having no material adverse effects, as defined in the agreement, with respect to the business and operations of the Corporation. See note 1.
The number of shares to be issued in connection with each notice shall be equal to the amount specified in the notice, divided by 97% of the average price of the Corporation’s common shares for the five days preceding the giving of the notice. The maximum amount of each notice is $1,000,000 and the minimum amount is $100,000. The Corporation may terminate the agreement before the 24-month term, if it has issued at least $8,000,000 of common shares under the agreement.
In the three-month period ended March 31, 2015, the Corporation issued nil (2014 – 258,924; 2013 – 368,035) common shares to the Purchaser under the agreements for proceeds of nil (2014 - $1,450,000; 2013 - $2,100,000). At March 31, 2015, the Corporation can require the Purchaser to purchase up to $9,550,000 of common shares over the remaining 7 months of the agreement, provided the Corporation adheres to its covenants.
12
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 9. |
Share capital (continued): |
|
|
|
(a) |
Common Stock Private Purchase Agreement (continued): |
Subsequent to March 31, 2015, the Corporation issued 431,344 common shares to the Purchaser for additional proceeds of $600,000.
The Corporation records the equity transaction at the amount received.
In the first quarter of 2015, the Corporation completed two private placements for a total of $400,000, of which $200,000 was received in December 2014 and classified as share capital subscription pending the closing of the transaction. A total of 883,058 Units were issued at an average price of $0.45 per unit. Each Unit is comprised of one common share and one-half of one common share purchase warrant (each whole warrant, a “Warrant”). Each Warrant entitles the holder to acquire one common share of the Corporation at a price per share equal to $2.00 for a period 24 months following the subscription date. A total of 883,058 shares and 441,529 warrants were issued, of which $341,520 of the total proceeds were attributed to the common shares based on the share price of the common shares of the date of the agreement and the remainder was attributed to the warrants.
The Corporation has established a stock option plan (the “Plan”) for its key employees, its officers and directors, and certain consultants. The Plan is administered by the Board of Directors of the Corporation. The Board may from time to time designate individuals to whom options to purchase common shares of the Corporation may be granted, the number of shares to be optioned to each, and the option price per share. The option price per share cannot involve a discount to the market price at the time the option is granted. The maximum number of shares which may be optioned under the stock option plan is 7,500,000. The maximum number of shares which may be optioned to any one individual is 15% of the total issued and outstanding common shares. Options under the Plan expire ten years after the grant date and vest either immediately or over periods up to six years, and are equity-settled. In the first quarter of 2015, 725,000 stock options expired ninety days following termination of optionees’ employment with the Corporation and optionee’s ceasing to be a director of the Corporation. As at March 31, 2015, 2,395,500 options (2014 – 1,710,500; 2013 – 1,569,500) could still be granted by the Corporation.
The following table provides the activity of stock option awards during the three-month period ended March 31, 2015 and for options outstanding and exercisable at the end of the three-month period ended March 31, 2015, the weighted average exercise price and the weighted average years to expiration.
13
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 9. |
Share capital (continued): |
|
|
|
(c) |
Stock options (continued): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options outstanding |
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
Weighted |
|
|
average |
|
|
|
|
|
|
|
average |
|
|
remaining |
|
|
|
|
|
|
|
exercise |
|
|
contractual |
|
|
|
|
Number |
|
|
price |
|
|
life (in years) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2014 |
|
5,829,500 |
|
$ |
4.39 |
|
|
3.92 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Expired |
|
(725,000) |
|
|
4.95 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, March 31, 2015 |
|
5,104,500 |
|
$ |
4.30 |
|
|
3.59 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Options exercisable |
|
5,057,000 |
|
$ |
4.28 |
|
|
3.55 |
|
|
(d) |
Stock-based compensation: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
Stock options granted in 2011 |
$ |
1,119 |
|
$ |
2,610 |
|
$ |
4,848 |
|
|
Stock options granted in 2012 |
|
6,102 |
|
|
11,332 |
|
|
21,792 |
|
|
Stock options granted in 2014 |
|
9,330 |
|
|
1,406,243 |
|
|
- |
|
|
Total stock-based compensation expense recognized |
$ |
16,551 |
|
$ |
1,420,185 |
|
$ |
26,640 |
|
The stock-based compensation expense is disaggregated in the statements of operations and comprehensive loss as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
Stock-based compensation pertaining to general and administrative |
$ |
15,432 |
|
$ |
792,325 |
|
$ |
21,792 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation pertaining to research and development |
|
1,119 |
|
|
627,860 |
|
|
4,848 |
|
|
|
$ |
16,551 |
|
$ |
1,420,185 |
|
$ |
26,640 |
|
No options were granted during the three-month period ended March 31, 2015 (2014 –600,000 options having a weighted average fair value of $2.54 per option; 2013 – none granted).
14
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 9. |
Share capital (continued): |
|
|
|
(d) |
Stock-based compensation (continued): |
The fair value of the options granted during the three-month period ended March 31, 2015 was determined using the Black-Scholes pricing model using the following weighted average assumptions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Share price |
|
- |
|
$ |
5.98 |
|
|
- |
|
|
Exercise price |
|
- |
|
$ |
5.98 |
|
|
- |
|
|
Risk-free interest rate |
|
- |
|
|
1.19 % |
|
|
- |
|
|
Expected volatility |
|
- |
|
|
54.10 % |
|
|
- |
|
|
Expected option life in years |
|
|
|
|
4 |
|
|
|
|
|
Expected dividends |
|
- |
|
|
- |
|
|
- |
|
Expected volatility was estimated considering a five-year historic average share price volatility.
Expected dividends were determined to be nil, since it is the present policy of the Corporation to retain all earnings to finance operations.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants outstanding |
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
Weighted |
|
|
average |
|
|
|
|
|
|
|
average |
|
|
remaining |
|
|
|
|
|
|
|
exercise |
|
|
contractual |
|
|
|
|
Number |
|
|
price |
|
|
life (in years) |
|
|
Outstanding, |
|
|
|
|
|
|
|
|
|
|
December 31, 2014 |
|
107,000 |
|
$ |
0.54 |
|
|
2.71 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised |
|
- |
|
|
- |
|
|
- |
|
|
Granted |
|
441,529 |
|
|
2.00 |
|
|
1.90 |
|
|
Expired |
|
- |
|
|
- |
|
|
- |
|
|
Cancelled |
|
- |
|
|
- |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding, |
|
|
|
|
|
|
|
|
|
|
March 31, 2015 |
|
548,529 |
|
$ |
1.72 |
|
|
2.06 |
|
On December 16, 2014, in connection with the convertible notes private placement financing referred to in note 6, the Corporation issued 107,000 warrants to the placement agent as partial consideration for the placement fees. Each warrant entitles the holder to acquire one common share of the Corporation at an exercise price of $0.54 prior to December 16, 2017.
15
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
| 9. |
Share capital (continued): |
|
|
|
(e) |
Warrants (continued): |
In the first quarter of 2015, the Corporation issued 441,529 warrants in connection with two private placements referred to in note 9 (b). Each warrant entitles the holder to acquire one common share of the Corporation at an exercice price of $2.00 prior to January 23, 2017 for 191,529 warrants and prior to March 12, 2017 for 250,000 warrants.
On November 24, 2014, a shareholder of the Corporation, filed a proposed class action suit in the United States District Court, District of New Jersey, against the Corporation and the President and the CEO of the Corporation. The motion was heard on January 26, 2015, and was the first procedural step before any class action could be instituted. The plaintiff seeks certification of a class action on behalf of all persons, wherever they reside, who acquired the Corporation’s common stock between January 31, 2011 and November 2, 2014. The plaintiff alleges that certain of the Corporation’s disclosures failed to disclose material adverse facts that raised serious questions as to the ability to achieve significant results for NX-1207 in Phase 3 trials in light of difficulty of enrolling candidates, obtaining objective and measured results, and the placebo effect. On March 10, 2015, the Corporation was served with a class-action lawsuit. The Corporation believes that the allegations made against it in these actions are meritless and will vigorously defend the matter, although no assurance can be given with respect to the ultimate outcome of such proceedings. No provision has been recognized in these financial statements for this matter.
Taxes on income in the interim periods are accrued using the tax rate that would be applicable to expected total annual profit or loss. The Corporation recognized no income taxes in the statements of operations and comprehensive loss, as it has been incurring losses since inception. Furthermore, no deferred tax asset was recognized since the accumulated tax losses available to be carried forward and used to offset future taxable income are not considered probable of being realized.
16
|
| NYMOX PHARMACEUTICAL CORPORATION |
| Notes to Condensed Interim Consolidated Financial Statements, Continued |
| (Unaudited) |
| |
| Three-month periods ended March 31, 2015, 2014 and 2013 |
| |
| (in US dollars) |
| |
Weighted average number of common shares outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Issued common shares at January 1 |
|
35,872,445 |
|
|
34,672,157 |
|
|
33,572,442 |
|
|
Effect of shares issued |
|
400,533 |
|
|
125,145 |
|
|
107,044 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding - basic |
|
36,272,978 |
|
|
34,797,302 |
|
|
33,679,486 |
|
|
Dilutive impact of convertible notes |
|
2,007,505 |
|
|
- |
|
|
- |
|
|
Weighted average number of shares outstanding - diluted |
|
38,280,483 |
|
|
34,797,302 |
|
|
33,679,486 |
|
For the three months ended March 31, 2015, we excluded 5,104,500 stock options and 548,529 warrants from the diluted weighted average per share calculation as they were anti-dilutive (2014 – 5,789,500 stock options: 2013 – 5,930,500 stock options). All outstanding stock options and warrants could potentially be dilutive in the future.
Executive officers and directors participate in the Corporation’s stock option plan (see note 8 (b)). Executive officers are covered under the Corporation’s health plan.
Key management personnel compensation is comprised of:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Salaries |
$ |
103,299 |
|
$ |
188,252 |
|
$ |
170,443 |
|
|
Short-term employee benefits |
|
934 |
|
|
2,468 |
|
|
2,564 |
|
|
Stock-based compensation |
|
9,330 |
|
|
1,406,243 |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
113,563 |
|
$ |
1,596,963 |
|
$ |
173,007 |
|
Total honorariums to the independent directors of the Corporation for participating in Board and Committee meetings are $13,500 for the period ended March 31, 2015 (2014 - $17,500; 2013 -$16,000).
On April 23, 2015, at a special meeting of the shareholders of the Corporation, the shareholders approved the transfer of the Corporation’s head office and domicile from Quebec, Canada to the Bahamas.
Subsequent to the end of the quarter, the Corporation granted 1,425,000 options to executive directors and officers at an exercise price of $1.15; these options are contingent upon and cannot be exercised until the Corporation’s stock trades for five consecutive days at a price of $5.00 or greater. In addition, the Corporation amended the exercise price of all of its 5,104,500 outstanding options to $1.15.
17
Exhibit 99.2
CERTIFICATION
I, Paul Averback, President and CEO of Nymox Pharmaceutical Corporation, certify that:
| 1. |
I have reviewed this quarterly report for the period ended March 31, 2015 of Nymox Pharmaceutical Corporation; |
| |
|
| 2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| |
|
| 3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| |
|
| 4. |
The company’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a – 15(e) and 15d – 15(e)), and internal control over financial reporting (as defined in the Exchange Act Rules 13a–15(f) and 15d–15(f)) for the company based on the COSO 1992 framework and we have: |
a) designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with international financial reporting standards;
c) evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and
| 5. |
The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent function): |
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting.
Date: May 15, 2015
/s/ Paul Averback, MD
Paul Averback, MD
President and Chief Executive Officer
Nymox Pharmaceutical Corporation
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
I, Paul Averback, President and CEO of Nymox Pharmaceutical Corporation, do hereby certify that, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, the information contained in the Quarterly Report for the period ended March 31, 2015 of Nymox Pharmaceutical Corporation and filed with the Securities and Exchange Commission, fully complies with the requirements of Section 13 (a) or 15 (d) of the Securities Exchange Act of 1934 and the information contained in such report fairly presents, in all material respects, the financial condition and results of operations of Nymox Pharmaceutical Corporation
Date: May 15, 2015
/s/ Paul Averback, MD
Paul Averback, MD
President and Chief Executive Officer
Nymox Pharmaceutical Corporation
Exhibit 99.3
CERTIFICATION
I, Andre Monette, CFO of Nymox Pharmaceutical Corporation, certify that:
| 1. |
I have reviewed this quarterly report for the period ended March 31, 2015 of Nymox Pharmaceutical Corporation; |
| |
|
| 2. |
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| |
|
| 3. |
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the company as of, and for, the periods presented in this report; |
| |
|
| 4. |
The company’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a – 15(e) and 15d – 15(e)), and internal control over financial reporting (as defined in the Exchange Act Rules 13a–15(f) and 15d–15(f)) for the company based on the COSO 1992 framework and we have: |
a) designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with international financial reporting standards;
c) evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the company’s internal control over financial reporting; and
| 5. |
The company’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit committee of the company’s board of directors (or persons performing the equivalent function): |
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting.
Date: May 15, 2015
/s/ Andre Monette
Andre Monette
Chief Financial Officer
Nymox Pharmaceutical Corporation
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
I, Andre Monette, CFO of Nymox Pharmaceutical Corporation, do hereby certify that, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, the information contained in the Quarterly Report for the period ended March 31, 2015 of Nymox Pharmaceutical Corporation and filed with the Securities and Exchange Commission, fully complies with the requirements of Section 13 (a) or 15 (d) of the Securities Exchange Act of 1934 and the information contained in such report fairly presents, in all material respects, the financial condition and results of operations of Nymox Pharmaceutical Corporation
Date: May 15, 2015
/s/ Andre Monette
Andre Monette
Chief Financial Officer
Nymox Pharmaceutical Corporation
Nymox Pharmaceutical (NASDAQ:NYMX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Nymox Pharmaceutical (NASDAQ:NYMX)
Historical Stock Chart
From Apr 2023 to Apr 2024