Use these links to rapidly review the document
TABLE OF CONTENTS
TABLE OF CONTENTS
Table of Contents
Filed Pursuant to Rule 424(b)(5)
Registration File No. 333-206525
PROSPECTUS SUPPLEMENT
(To Prospectus dated August 23, 2016)
18,400,000 shares

Common stock
We are offering 18,400,000 shares of our common stock.
Our
common stock is quoted on The NASDAQ Global Market under the symbol "ARRY." The last reported sale price of our common stock on The NASDAQ Global Market was $6.59 per share on September 27,
2016.
Investing in our common stock involves a high degree of risk. See "Risk factors" beginning on page S-10 of this prospectus supplement and in the documents incorporated
by reference into this prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement
or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Price share
|
|
Total
|
|
|
|
|
|
|
|
|
|
|
|
Public offering price
|
|
$
|
6.25
|
|
$
|
115,000,000
|
|
|
Underwriting discounts and commissions(1)
|
|
$
|
0.375
|
|
$
|
6,900,000
|
|
|
Proceeds, before expenses, to us
|
|
$
|
5.875
|
|
$
|
108,100,000
|
|
|
|
|
|
|
|
|
|
|
(1) We
refer you to the "Underwriting" section of this prospectus supplement for additional information regarding underwriter compensation.
We
have granted the underwriters the right to purchase up to an additional 2,760,000 shares of our common stock at the public offering price less the underwriting discounts and commissions. The
underwriters can exercise this right at any time within 30 days after the date of this prospectus supplement.
The
underwriters expect to deliver the shares to the investors on or about October 3, 2016.
|
|
|
|
|
|
|
J.P. Morgan
|
|
|
|
Cowen and Company
|
Stifel
|
|
|
|
Wells Fargo Securities
|
SunTrust Robinson Humphrey
|
The date of this prospectus supplement is September 27, 2016.
Table of Contents
Table of contents
Prospectus supplement
Prospectus
S-i
Table of Contents
About this prospectus supplement
This document is part of the registration statement that we filed with the Securities and Exchange Commission, or the SEC, using a
"shelf" registration process and consists of two parts. The first part is this prospectus supplement, which describes the terms of this offering of common stock and also adds to and updates
information contained in the accompanying prospectus and the documents incorporated by reference. The second part is the accompanying prospectus, which gives more general information, some of which
may not apply to this offering of common stock. To the extent the information contained in this prospectus supplement differs or varies from the information contained in the accompanying prospectus or
any document incorporated by reference, the information in this prospectus supplement shall control.
You
should rely only on the information contained or incorporated by reference in this prospectus supplement, the accompanying prospectus and any free writing prospectus that we authorize to be
distributed to you. We have not, and the underwriters have not, authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you
should not rely on it. We are not, and the underwriters are not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the
information appearing in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and any free
writing prospectus is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You should
read this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and any free writing prospectus
when making your investment decision. You should also read and consider the information in the documents we have referred you to in the sections of this prospectus supplement entitled "Information
Incorporated by Reference" and of the prospectus entitled "Where you can find more information" and "Incorporation of certain information by reference."
References
in this prospectus supplement to "Array," "the company," "we," "our" or "us" refer to Array BioPharma Inc. Our trademarks include the Array BioPharma logo and the term "Array
BioPharma". Other brand names, trademarks and trade names appearing in this prospectus supplement and the accompanying prospectus are the property of the respective holders of such trademarks and
trade names.
S-1
Table of Contents
Prospectus supplement summary
This summary highlights selected information contained elsewhere or incorporated by reference in this
prospectus supplement. This summary does not contain all of the information that you should consider before investing in our common stock. Before making an investment in our common stock, you should
read this entire prospectus supplement and the accompanying prospectus carefully, including the risk factors described under the heading "Risk Factors" in this prospectus supplement starting on
page S-10, in our Annual Report on Form 10-K for the fiscal year ended June 30, 2016 and the financial statements and other information incorporated by reference in this
prospectus supplement and the accompanying prospectus. Unless the context otherwise requires, any reference to "Array," "we," "our" and "us" in this prospectus supplement refers to Array
BioPharma Inc. and its subsidiary.
Our business
Array is a biopharmaceutical company focused on the discovery, development and commercialization of targeted small molecule drugs to
treat patients afflicted with cancer. Five registration studies are currently advancing related to three cancer drugs. These programs include three cancer drugs, binimetinib (MEK162), encorafenib
(LGX818) and selumetinib (partnered with AstraZeneca).
Our
most advanced clinical stage drugs include:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Drug candidate
|
|
Target/Indication
|
|
Partner
|
|
Clinical
status
|
|
|
|
|
|
|
|
|
|
Binimetinib
|
|
MEK inhibitor for cancer
|
|
Pierre Fabre
|
|
Phase 3
|
|
Encorafenib
|
|
BRAF inhibitor for cancer
|
|
Pierre Fabre
|
|
Phase 3
|
|
Filanesib
|
|
Kinesin spindle protein, or KSP, inhibitor for multiple myeloma
|
|
|
|
Phase 2
|
|
ARRY-797
|
|
p38 inhibitor for Lamin A/C-related dilated cardiomyopathy
|
|
|
|
Phase 2
|
|
Selumetinib
|
|
MEK inhibitor for cancer
|
|
AstraZeneca, PLC
|
|
Phase 3
|
|
ASC08/Danoprevir
|
|
Protease inhibitor for Hepatitis C virus
|
|
Roche Holding AG
|
|
Phase 3
|
|
ASLAN001/Varlitinib
|
|
Pan-HER2 inhibitor for gastric or breast cancer
|
|
ASLAN Pharmaceuticals Pte Ltd.
|
|
Phase 2
|
|
Ipatasertib/GDC-0068
|
|
AKT inhibitor for cancer
|
|
Genentech, Inc.
|
|
Phase 2
|
|
Motolimod/VTX-2337
|
|
Toll-like receptor for cancer
|
|
VentiRx Pharmaceuticals, Inc.
|
|
Phase 2
|
|
Prexasertib/LY2606368
|
|
Chk-1 inhibitor for cancer
|
|
Eli Lilly and Company
|
|
Phase 2
|
|
LOXO-101
|
|
PanTrk inhibitor for cancer
|
|
Loxo Oncology, Inc.
|
|
Phase 2
|
|
ONT-380/ARRY-380
|
|
HER2 inhibitor for breast cancer
|
|
Cascadian Therapeutics
|
|
Phase 2
|
|
GDC-0575
|
|
Chk-1 inhibitor for cancer
|
|
Genentech, Inc.
|
|
Phase 1b
|
|
GDC-0994
|
|
ERK inhibitor for cancer
|
|
Genentech, Inc.
|
|
Phase 1
|
|
ARRY-382
|
|
CSF1R inhibitor for cancer
|
|
|
|
Phase 1
|
|
|
|
|
|
|
|
|
S-2
Table of Contents
Binimetinib and encorafenib
In March 2015, Array regained development and commercialization rights to binimetinib and acquired development and commercialization rights to
encorafenib under the Termination and Asset Transfer Agreement with Novartis Pharma AG and Novartis Pharmaceutical Ltd. and the Asset Transfer Agreement with Novartis Pharma AG, collectively,
referred to herein as the Novartis Agreements. Along with global ownership of both assets, Array received an up-front payment of $85.0 million from Novartis. We believe these programs present
significant opportunity to Array in the area of oncology.
We
have also entered into a Development and Commercialization Agreement with Pierre Fabre Medicament SAS, or Pierre Fabre, which became effective in December 2015, or the PF Agreement, pursuant to
which we granted Pierre Fabre rights to commercialize binimetinib and encorafenib in all countries except for the United States, Canada, Japan, Korea and Israel, where Array will retain its ownership
rights. The PF Agreement satisfied our commitment to secure a development and commercialization partner for the
European market for both encorafenib and binimetinib acceptable to European Commission regulatory agencies made in connection with the Novartis Agreements.
All
clinical trials involving binimetinib and encorafenib that were active or planned when the Novartis Agreement became effective in March 2015, including the NEMO and COLUMBUS trials and other then
active Novartis-sponsored and investigator-sponsored clinical studies, continue to be reimbursed pursuant to the terms of the Novartis Agreements. Further worldwide development activities of
binimetinib and encorafenib will be governed by a Global Development Plan, or GDP, with Pierre Fabre. Pierre Fabre and Array will each have the option to jointly fund worldwide development costs under
the GDP, with Array covering 60% and Pierre Fabre covering 40% of such costs. The initial GDP includes multiple trials, including the Phase 3 BEACON colorectal cancer, or CRC, trial, and Pierre
Fabre and Array have agreed to commit at least €100 million in combined funds for these studies.
Pierre
Fabre is responsible for seeking regulatory and pricing and reimbursement approvals in the European Economic Area and its other licensed territories. We have also agreed to enter into a
clinical and commercial supply agreement with Pierre Fabre pursuant to which we will supply or procure the supply of clinical and commercial supplies of drug substance and drug product for Pierre
Fabre, the costs of which will be borne by Pierre Fabre. We have also agreed to cooperate with Pierre Fabre to ensure the supply of companion diagnostics for use with binimetinib and encorafenib in
indications where needed.
Binimetinib
and encorafenib are currently being studied in Phase 3 trials in advanced cancer patients, including the NEMO trial studying binimetinib in patients with NRAS-mutant melanoma, the
COLUMBUS trial (Combined LGX818 Used with MEK162 in BRAF-Mutant Unresectable Skin Cancer) studying encorafenib in combination with binimetinib in patients with BRAF-mutant melanoma and the
recently-initiated BEACON trial (Binimetinib, Encorafenib And Cetuximab Combined to treat BRAF-mutant Colorectal Cancer) studying encorafenib in combination with binimetinib and cetuximab in patients
with BRAF V600E-mutant colorectal cancer, or BRAFm CRC.
COLUMBUS
The COLUMBUS trial is a two-part, international, randomized, open label Phase 3 study evaluating the efficacy and safety of the combination of
encorafenib and binimetinib as compared to vemurafenib and encorafenib monotherapy in 921 patients with locally advanced unresectable or metastatic melanoma with
BRAF
V600
mutation. Prior immunotherapy treatment was allowed. Over 200 sites across North America,
S-3
Table of Contents
Europe,
South America, Africa, Asia and Australia participated in the study. Patients were randomized into two parts:
-
•
-
In Part 1, 577 patients were randomized 1:1:1 to receive the combination of 450mg encorafenib and 45mg binimetinib, 300mg
encorafenib alone, or 960mg vemurafenib alone. The primary endpoint for the COLUMBUS trial is progression free survival, or PFS, comparison of the combination vs. vemurafenib. PFS is determined based
on tumor assessment (RECIST version 1.1 criteria) by a Blinded Independent Review Committee. Secondary endpoints include a comparison of the PFS of encorafenib monotherapy to that of the combination
and a comparison of overall survival, or OS, for the combination to that of vemurafenib alone.
-
•
-
In Part 2, 344 patients were randomized 3:1 to receive 300mg encorafenib plus 45mg binimetinib or 300mg encorafenib alone.
Part 2 is designed to provide additional data to help evaluate the contribution of binimetinib to the combination. While formal statistical analysis of Part 2 is only planned if both the
comparison of PFS between combination vs. vemurafenib and the combination vs. encorafenib achieve statistical significance in Part 1, data from Part 2 are anticipated in mid-2017 and it
is expected to be provided to global health authorities as supportive evidence for planned regulatory submissions in 2017.
On
September 26, 2016, Array announced top-line results from Part 1 of the COLUMBUS trial. The trial met its primary endpoint with median PFS for patients treated with the combination of
encorafenib plus binimetinib, or combination, of 14.9 months versus 7.3 months for patients treated with vemurafenib; [hazard ratio (HR) = 0.54, (95% CI, 0.41-0.71),
p<0.001].
The
combination was generally well-tolerated and reported adverse events for the combination were overall consistent with previous clinical trial results of encorafenib plus binimetinib combination
trial results in
BRAF
-mutant melanoma patients.
Analysis
of a secondary endpoint comparing the PFS of patients treated with combination to patients treated with encorafenib showed a median of 14.9 months versus 9.6 months
[HR = 0.75, (95% CI, 0.56-1.00), p=0.051], which did not reach statistical significance.
We
anticipate that additional results from Part 1 of the COLUMBUS trial, including objective response rates, disease control rates, safety endpoints, exploratory analyses such as a
Part 1 PFS comparison of encorafenib to vemurafenib and pre-specified subgroup analyses including outcomes in patients who received prior treatment with immunotherapy, will be presented at an
upcoming medical meeting. Analysis of the secondary endpoint of OS was not planned as part of these initial results.
Based
on reported sales of approved MEK/BRAF combination agents, we believe that annual worldwide sales for such MEK/BRAF combination agents is approaching $1 billion, with U.S. sales
approaching $350 million.
BEACON
In June 2016, Array, Pierre Fabre and Merck KGaA, Darmstadt, Germany, jointly initiated the BEACON CRC trial. BEACON CRC is a randomized, open-label,
global Phase 3 clinical trial designed to assess the safety and efficacy of binimetinib, encorafenib and Erbitux® (cetuximab), a monoclonal antibody, in comparison to Erbitux and
irinotecan-based therapy in patients with BRAFm CRC who have previously received first- or second-line systemic therapy. The study includes a safety lead-in with approximately 30 patients.
S-4
Table of Contents
With
appropriate results from the lead-in, approximately 615 patients are expected to be randomized 1:1:1 to receive triplet therapy (binimetinib, encorafenib and Erbitux), doublet therapy
(encorafenib and Erbitux) or the control arm (irinotecan-based therapy and Erbitux).
The
primary endpoint of the trial is OS of the triplet therapy compared to the control arm. The secondary endpoints address efficacy of the doublet therapy compared to the control arm, and the triplet
therapy compared to the doublet therapy. Other secondary endpoints include PFS, objective response rate, or ORR, duration of response, safety and tolerability. Health related quality of life data will
also be assessed. The trial will be conducted at over 250 investigational sites in North America, South America, Europe and the Asia Pacific region. Patient enrollment is expected to be completed in
2018.
Array
will act as the global sponsor of the study. Pursuant to the PF Agreement with Pierre Fabre, Pierre Fabre has elected to co-fund 40% of the cost of the BEACON CRC trial. Merck KGaA, Darmstadt,
Germany, is the owner of Erbitux outside the United States and Canada, and will supply Erbitux to all trial sites outside the United States and Canada as part of the collaboration. If successful,
results would support regulatory submissions for all three parties.
On
September 14, 2016, Array announced that it reached agreement with the U.S. Food and Drug Administration, or FDA, regarding a Special Protocol Assessment, or SPA, related to BEACON. The SPA
provides agreement that the design and planned analysis of the BEACON trial adequately address the objectives necessary to support a regulatory submission for the approval of the doublet regiment of
encorafenib and Erbitux. The FDA separately communicated that if the triplet regimen both met its primary endpoint (OS) as compared to the control arm and demonstrated a clinically meaningful benefit
as compared to the doublet regimen, the study would provide support for approval of the triplet regimen.
The
BEACON CRC trial was initiated following completion of a Phase 2 study of the combination of encorafenib and cetuximab, with or without alpelisib, a selective PI3K alpha inhibitor, in
patients with advanced BRAFm CRC. In June 2016, Array announced that data from this study showed that median OS for these patients exceeded one year, which is more than double several historical
published benchmarks for this population.
In
the Phase 2 study, 102 patients with BRAFm CRC who had progressed after one or more prior therapies were randomized to receive the doublet regimen of encorafenib and cetuximab (ENCO 200 mg
PO QD and CETUX per label; n=50) or the triplet regimen of encorafenib, cetuximab and alpelisib (ENCO, CETUX and
ALP 300 mg PO QD; n=52). The Phase 2 data for these treatment regimens showed promising clinical activity in patients:
-
•
-
Median OS was 12.4 and 13.1 months for the doublet and alpelisib-containing triplet regimens, respectively.
-
•
-
Median PFS was 4.2 and 5.4 months for the doublet and alpelisib-containing triplet regimens, respectively.
-
•
-
ORR was 22 percent and 27 percent for the doublet and alpelisib-containing triplet regimens, respectively.
-
•
-
Grade 3 or 4 adverse events occurred in greater than 10 percent of patients and included anemia, hyperglycemia and increased
lipase.
Historical
results from other published studies show median PFS and median OS after first-line treatment for BRAFm CRC patients range from 1.8 to 2.5 months and four to six months,
respectively, and published
S-5
Table of Contents
ORR
from various studies in this population range between six percent to eight percent. The study showed that alpelisib added toxicity with only marginal additional activity, therefore the BEACON CRC
trial will replace it with binimetinib in the triplet arm.
Colorectal
cancer is the third most common cancer among men and women in the United States, with more than 134,000 new cases and nearly 50,000 deaths from the disease projected in 2016. In the United
States, BRAF mutations occur in 8 to 15 percent of patients with colorectal cancer and the prognosis for these patients is poor.
NEMO
NEMO is a Phase 3 study comparing binimetinib versus dacarbazine in unresectable or metastatic NRAS-mutant melanoma patients. On
September 1, 2016, Array announced that the FDA had accepted Array's New Drug Application, or NDA, for binimetinib in patients with advanced NRAS-mutant melanoma with a target action date under
the Prescription Drug User Fee Act (PDUFA) of June 30, 2017. The FDA also indicated that it plans to hold an Oncologic Drugs Advisory Committee (ODAC) meeting as part of the regulatory process.
Activating
NRAS mutations are present in approximately 20% of patients with metastatic melanoma, and have been a poor prognostic indicator for these patients. Treatment options for this population
remain limited beyond immunotherapy (PD-1, CTLA4). Therefore, if approved, binimetinib could represent an important additional therapy for these patients.
The
NDA submission is based on results of the NEMO study, which found binimetinib extended median PFS, the study's primary endpoint, as compared with dacarbazine. In the NEMO study, binimetinib
extended median PFS at 2.8 months, as compared with 1.5 months observed with dacarbazine [hazard ratio (HR)=0.62 (95% CI, 0.47-0.80), p<0.001] in
patients with advanced NRAS-mutant melanoma. In the pre-specified subset of patients who received prior treatment with immunotherapy (n=85), including ipilimumab (n=54), and nivolumab or pembrolizumab
(n=24), patients who received binimetinib experienced 5.5 months of median PFS (95% CI, 2.8-7.6), compared with 1.6 months for those receiving treatment with dacarbazine (95% CI,
1.5-2.8). While the results in the pre-specified sub-group of patients who had received prior treatment with immunotherapy are of interest, interpretation beyond overall consistency with the primary
result should be made with care. Array anticipates that the primary consideration for marketing approval will be the results for the primary endpoint of the trial.
In
addition to improving PFS, binimetinib also demonstrated significant improvement in the secondary endpoints of ORR and disease control rate, or DCR. While there was no statistically significant
difference demonstrated in overall survival, the numerical trend in median overall survival, or mOS, favored the binimetinib arm.
-
•
-
Confirmed ORR was 15 percent (95% CI, 11-20 percent) in patients receiving binimetinib vs. 7 percent (95% CI,
3-13 percent) in patients receiving dacarbazine.
-
•
-
Confirmed ORR for patients who received prior treatment with immunotherapy was 16 percent, with a median duration of response
of 11.1 months, versus an overall response rate for all patients receiving binimetinib of 15 percent, with a median duration of response of 6.9 months.
-
•
-
DCR for patients receiving binimetinib was 58 percent (95% CI, 52-64) vs. 25 percent (95% CI, 18-33 percent) for
patients receiving dacarbazine.
S-6
Table of Contents
-
•
-
mOS was estimated at 11.0 months in patients receiving binimetinib vs. 10.1 months for patients treated with dacarbazine
[(HR) = 1.0 (95% CI 0.75-1.33), p=0.499].
Binimetinib
was generally well-tolerated and the adverse events reported were consistent with previous results in NRAS-mutant melanoma patients. Grade
3
/
4
adverse events reported in at
least 5 percent of patients receiving binimetinib included increased creatine phosphokinase (CPK) and hypertension.
Following
discontinuation of the study treatment, a substantial portion of the patients in the study were treated with immunotherapy, including 46% of the patients who had received binimetinib in the
study and 44% of the patients who had received dacarbazine. Specifically, 33% of the former binimetinib patients received ipilimumab, 13% of such patients received nivolumab and 12% of such patients
received pembrolizumab. Of the former patients who received dacarbazine, 36% of such patients received ipilimumab, 9% received nivolumab and 7% received pembrolizumab.
The
NEMO results were presented at the 2016 American Society of Clinical Oncology (ASCO) Annual Congress in June 2016.
MILO
On April 1, 2016, we announced our decision to discontinue the MILO study, a Phase 3 trial of binimetinib for the treatment of patients
with low grade serous ovarian cancer. The decision to stop the study was made after a planned interim analysis showed that the Hazard Ratio for Progression Free Survival crossed the predefined
futility boundary.
Selumetinib
AstraZeneca continues to advance selumetinib in two registration trials: a trial in patients with neurofibromatosis type 1 and the ASTRA trial
in patients with differentiated thyroid cancer. AstraZeneca estimates top-line results from both trials in 2017. In August 2016, AstraZeneca announced results from the Phase 3 SELECT-1 trial of
selumetinib in combination with docetaxel chemotherapy as 2nd-line treatment in patients with KRAS mutation-positive locally-advanced or metastatic non-small cell
lung cancer. The results showed that the trial did not meet its primary endpoint of progression-free survival and selumetinib did not have a significant effect on overall survival. The adverse event
profiles for selumetinib and docetaxel were consistent with those previously reported.
ARRY-797
Array conducted a 12-patient Phase 2 study to evaluate the effectiveness and safety of ARRY-797 in patients with LMNA A/C-related DCM, a
serious, genetic cardiovascular disease. By age 45, approximately 70% of patients with LMNA A/C-related DCM will have died, suffered a major cardiac event, or will have undergone a heart transplant.
The primary endpoint of the trial was mean change in 6-minute walk test, or 6MWT, at 12 weeks relative to baseline. The trial results were presented at the European Society of Cardiology
meeting on August 30, 2016. The results demonstrated an absolute mean change from baseline of 69 meters on the 6MWT at week 12, the study's primary endpoint (baseline 6MWT ranged from 246 to
412 meters). This magnitude of improvement exceeded historical benchmarks for 6MWT that has served as the basis for recent approvals of drugs in other rare diseases. ARRY-797 administration also
resulted in sustained improvements in N-terminal pro-brain natriuretic peptide, or NT-proBNP, functional capacity and cardiac function through 48 weeks in LMNA-related DCM patients. Patients
who rolled over to a continuing treatment protocol maintained improvements in the 6MWT and NT-proBNP levels through 72 weeks of treatment. Other secondary endpoints measured including
echocardiographic measures of left and right ventricular function and patient-reported outcomes using the Kansas City Cardiomyopathy Questionnaire,
S-7
Table of Contents
both
mirrored the favorable improvements seen with the 6MWT. Taken together, the data to date suggest a path forward for this program, and Array has met with regulators to discuss the design of a
study that could be the basis for marketing approval.
ARRY-382
In July 2016, Array initiated a Phase
1
/
2
dose escalation immuno-oncology trial of ARRY-382 in combination with pembrolizumab
(Keytruda®), a Programmed Cell Death Receptor 1 (PD-1) antibody, in patients with advanced solid tumors. ARRY-382 is a potent, highly selective, small-molecule inhibitor of CSF-1R kinase
activity that is wholly-owned by Array.
The
study will enroll up to 18 patients with selected advanced solid tumors to determine the maximum tolerated dose and/or recommended Phase 2 dose of the combination. In addition, the safety
profile, pharmacodynamic effects, and preliminary assessment of activity of the combination will be assessed. With appropriate results, Array has the option to advance the combination into expansion
cohorts of patients with metastatic melanoma or advanced non-small cell lung cancer.
Results
from a prior Phase 1 study designed to assess the safety, pharmacokinetics and pharmacodynamics of ARRY-382 for treating patients with cancer have been presented and a dose and schedule
that demonstrates target engagement based on multiple pharmacodynamic biomarkers was identified for further study.
Filanesib
Given Array's significant opportunity with the Phase 3 binimetinib and encorafenib programs across a number of cancer indications, Array
currently has no plans to initiate additional trials with filanesib, a highly selective, targeted KSP inhibitor. Two studies in patients with relapsed / refractory multiple myeloma are nearing
completion: a randomized Phase 2 trial of the combination of filanesib and Kyprolis® (carfilzomib) and Kyprolis alone (ARRAY-520-216) and the AfFIRM trial, a Phase 2 single
agent study.
We
also have a portfolio of proprietary and partnered preclinical drug discovery programs.
Our corporate information
Our principal executive offices are located at 3200 Walnut Street, Boulder, Colorado 80301 and our phone number is
(303) 381-6600. We were founded in 1998 and became a public company in November 2000. We also maintain a web site at http://www.arraybiopharma.com. Our website and the information contained on,
or that can be accessed through, the website will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus supplement or accompanying prospectus. You should
not rely on any such information in making your decision whether to purchase our common stock.
S-8
Table of Contents
The offering
|
|
|
|
|
Common stock offered by us
|
|
18,400,000 shares
|
Common stock to be outstanding after this offering
|
|
162,090,104 shares
|
Option to purchase additional shares
|
|
We have granted the underwriters an option to purchase up to an additional 2,760,000 shares of common stock. The underwriters can exercise this option at any time within 30 days from the date of
this prospectus.
|
Use of proceeds
|
|
We intend to use the net proceeds from this offering to fund our research and development efforts, including clinical trials for our proprietary candidates, build and scale commercial capability, and
for general corporate purposes, including general working capital purposes. See "Use of proceeds."
|
The NASDAQ Global Market symbol
|
|
ARRY
|
Risk factors
|
|
See "Risk factors" and the other information included in, or incorporated by reference into, this prospectus supplement and the accompanying prospectus for a discussion of factors you should carefully
consider before deciding to invest in shares of our common stock.
|
The
number of shares of our common stock to be outstanding after this offering is based on 143,690,104 shares of common stock outstanding as of June 30, 2016 and
excludes:
-
•
-
11,647,595 shares of common stock issuable upon exercise of options outstanding as of June 30, 2016 at a weighted average
exercise price of $4.80 per share;
-
•
-
832,100 shares of common stock reserved for issuance upon vesting of restricted stock units as of June 30, 2016;
-
•
-
19,228,677 shares of common stock available for future issuance under our Amended and Restated Stock Option and Incentive Plan as of
June 30, 2016;
-
•
-
586,104 shares of common stock available for future issuance under our Amended and Restated Employee Stock Purchase Plan as of
June 30, 2016; and
-
•
-
18,761,527 shares of common stock issuable upon conversion of our 3.00% Convertible Senior Notes due in 2020.
Unless
otherwise indicated, all information in this prospectus supplement assumes that the underwriters will not exercise the option to purchase additional shares granted to them by us.
S-9
Table of Contents
Risk factors
Investment in our common stock involves a high degree of risk. In addition to the other information included
or incorporated by reference in this prospectus supplement and the accompanying prospectus, you should carefully consider the specific risks set forth below before making an investment decision. The
risks and uncertainties described below could adversely affect our business, operating results and financial condition, as well as cause the value of our common stock to decline. You may lose all or
part of your investment as a result. You should also refer to the other information contained in this prospectus supplement and the accompanying prospectus or incorporated by reference, including our
financial statements and the notes to those statements, and the information set forth under the caption "Forward-Looking Statements."
Risks related to our stock and this offering
Our quarterly operating results could fluctuate significantly, which could cause our stock price to
decline.
Our quarterly operating results have fluctuated in the past and are likely to fluctuate in the future. Entering into licensing or drug discovery
collaborations typically involves significant technical evaluation and/or commitment of capital by our collaborators. Accordingly, negotiation can be lengthy and is subject to a number of significant
risks, including budgetary constraints and internal acceptance reviews. In addition, we have no approved drugs on the market that generate recurring royalty income, and a significant portion of our
revenue is attributable to up-front and milestone payments. The achievement of milestones that trigger milestone payments occurs infrequently and the payments are non-recurring. In addition, even if a
collaboration yields a commercial product, the products sales on which royalties would be based may be small or insignificant. Further, some of our collaborators can influence the timing of the
achievement of milestones and when we deliver products and perform services, and therefore receive revenue, under their contracts with us making it difficult to predict or control our revenue. Due to
these factors, our operating results could fluctuate significantly from quarter to quarter. In addition, we may experience significant fluctuations in quarterly operating results due to factors such
as general and industry-specific economic conditions that may affect the research and development expenditures of pharmaceutical and biotechnology companies.
Due
to the possibility of fluctuations in our revenue and expenses, we believe that quarter-to-quarter comparisons of our operating results are not a good indication of our future performance. Our
operating results in some quarters may not meet the expectations of stock market analysts and investors. If we do not meet analysts' and/or investors' expectations, our stock price could decline.
Because our stock price may be volatile, our stock price could experience substantial declines.
The market price of our common stock has historically experienced and may continue to experience volatility. The high and low sales price for our
common stock were $7.31 and $2.38, respectively, in fiscal 2016 and $8.59 and $2.98, respectively, in fiscal 2015. Our quarterly operating results, the success or failure of our internal drug
discovery efforts, developments or disputes concerning our patents or proprietary rights, changes in general conditions in the economy or the financial markets and other developments affecting our
collaborators, our competitors or us could cause the market price of our common stock to fluctuate substantially. This volatility coupled with market declines in our industry over the past several
years have affected the market prices of securities issued by many companies, often for reasons unrelated to their operating performance, and may adversely affect the price of our common stock. In the
past, securities class action litigation has often been instituted following periods of volatility
S-10
Table of Contents
in
the market price of a company's securities. A securities class action suit against us could result in potential liabilities, substantial costs and the diversion of management's attention and
resources, regardless of whether we win or lose.
Because we do not intend to pay dividends, stockholders will benefit from an investment in our
common stock only if it appreciates in value.
We have never declared or paid any cash dividends on our common stock and are restricted in our ability to do so under our current credit agreement.
We currently intend to retain our future earnings, if any, to finance the expansion of our business and do not expect to pay any cash dividends in the foreseeable future. As a result, the success of
an investment in our common stock will depend entirely upon any future appreciation. There is no guarantee that our common stock will appreciate in value or even maintain the price at which
stockholders have purchased their shares.
The ability of our stockholders to control our policies and effect a change of control of our
company is limited, which may not be in the best interests of our stockholders.
There are provisions in our certificate of incorporation and bylaws that may discourage a third party from making a proposal to acquire us, even if
some of our stockholders might consider the proposal to be in their best interests. These include the following provisions in our certificate of
incorporation:
-
•
-
Our certificate of incorporation provides for three classes of directors with the term of office of one class expiring each year,
commonly referred to as a "staggered board." By preventing stockholders from voting on the election of more than one class of directors at any annual meeting of stockholders, this provision may have
the effect of keeping the current members of our board of directors in control for a longer period of time than stockholders may desire.
-
•
-
Our certificate of incorporation authorizes our board of directors to issue shares of preferred stock without stockholder approval and
to establish the preferences and rights of any preferred stock issued, which would allow the board to issue one or more classes or series of preferred stock that could discourage or delay a tender
offer or change in control.
We
are also subject to the business combination provisions of Section 203 of the Delaware General Corporation Law, which, in general, imposes restrictions upon acquirers of 15% or more of our
stock. As a result, it is difficult for a third party to acquire control of us without the approval of the board of directors and, therefore, mergers and acquisitions of us that our stockholders may
consider in their best interests may not occur.
Future sales of our common stock may depress our stock price.
The market price of our common stock could decline as a result of sales of substantial amounts of our common stock in the public market after the
closing of this offering, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of common stock. There
were 143,690,104 shares of common stock outstanding as of June 30, 2016. All of the shares sold in this offering will be freely transferable without restriction or further registration under
the Securities Act of 1933.
In
addition, future issuances by us of shares of our common stock upon the exercise or settlement of equity-based awards would dilute existing stockholders' ownership interests in our company, and any
sales in the public market of these shares, or the perception that these sales might occur, could also adversely affect the market price of shares of our common stock.
S-11
Table of Contents
In
the future, we may offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock in order to raise additional capital. We cannot assure you
that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering. Investors
purchasing shares or other securities in the future could have rights, preferences or privileges senior to those of existing stockholders and you may experience dilution. You may incur additional
dilution upon the exercise of any outstanding stock options or settlement of restricted stock units.
We have broad discretion in the use of the net proceeds from this offering, and we may not use these
proceeds effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not
necessarily improve our results of operations or enhance the value of our common stock. We intend to use the net proceeds from
this offering to fund our research and development efforts, including clinical trials for our proprietary candidates, build and scale commercial capability, and for general corporate purposes,
including general working capital purposes. However, our use of these net proceeds may differ substantially from our current plans and our management might not apply our net proceeds in ways that
ultimately increase the value of your investment. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our
business or financial condition, cause the price of our common stock to decline and delay product development.
You will experience immediate dilution in the book value per share of the common stock you purchase.
Since the price per share of our common stock being offered is substantially higher than the book value per share of our common stock, you will
suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering. After giving effect to the sale of 18,400,000 shares of our common stock in this offering
at the public offering price of $6.25 per share, if you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of approximately $5.82 per share in the net
tangible book value of the common stock. See the section entitled "Dilution" below for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
A substantial number of shares of our outstanding common stock may be sold in this offering, which
could cause the price of our common stock to decline.
In this offering, assuming the underwriter's option to purchase up to 2,760,000 additional shares from us is exercised in full, we will sell
21,160,000 shares, or approximately 14.7% of our outstanding common stock as of June 30, 2016. This sale and any future sales of a substantial number of shares of our common stock in the public
market, or the perception that such sales may occur, could adversely affect the price of our common stock. We cannot predict the effect, if any, that market sales of those shares of common stock or
the availability of those shares of common stock for sale will have on the market price of our common stock.
We will need to raise additional capital to support our future operations, which may involve
financings that could result in substantial dilution to our stockholders and the creation of additional securities senior to our common stock.
The terms of this offering do not restrict our ability to offer additional shares of common stock or a new series of preferred stock. Our board of
directors has the authority to establish the designation of shares of preferred stock that may be convertible into common stock without any action by our stockholders, and to
S-12
Table of Contents
fix
the rights, preferences, privileges and restrictions, including voting rights, of such shares. Such shares of preferred stock may have rights, preferences and privileges senior to those of
outstanding common stock and the issuance and conversion of any such preferred stock would further dilute the percentage ownership of our stockholders.
S-13
Table of Contents
Forward-looking statements
This prospectus supplement, the accompanying prospectus, the documents we have filed with the Securities and Exchange Commission that
are incorporated by reference and any free writing prospectus that we have authorized for use in connection with this offering contain certain forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. All statements that are not descriptions of historical facts are forward-looking statements and can generally be identified by the use of
forward-looking terminology such as "believes," "expects," "intends," "may," "will," "should," or "anticipates" or similar terminology.
These
statements reflect our current expectations about future events, and, as a result, our actual results could differ materially from those anticipated in these forward-looking statements. The
factors that could cause actual results to differ from our expectations are described under the caption "Risk Factors" set forth on page S-10 of this prospectus supplement and in documents we
have filed with the Securities and Exchange Commission that are incorporated by reference. These forward-looking statements include, among other things, statements
regarding:
-
•
-
our ability to continue to fund and successfully progress internal research and development efforts and to create effective,
commercially viable drugs;
-
•
-
our ability to effectively and timely conduct clinical trials in light of increasing costs and difficulties in locating appropriate
trial sites and in enrolling patients who meet the criteria for certain clinical trials;
-
•
-
risks associated with our dependence on our collaborators for the clinical development and commercialization of our out-licensed drug
candidates;
-
•
-
the ability of our collaborators and of Array to meet objectives that must be achieved for Array to receive milestone payments and
royalties;
-
•
-
the extent to which the pharmaceutical and biotechnology industries are willing to in-license drug candidates for their product
pipelines and to collaborate with and fund third parties on their drug discovery activities;
-
•
-
our ability to out-license our proprietary candidates on favorable terms;
-
•
-
our ability to attract and retain experienced scientists and management;
-
•
-
our ability to achieve and maintain profitability; and
-
•
-
the use of proceeds from this offering.
All
forward-looking statements represent our judgment as of the date of the document containing the statement. We may not actually achieve the plans, intentions or expectations disclosed in our
forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations
disclosed in the forward-looking statements we make. We undertake no obligation to update any forward-looking statement to reflect new information or future events or developments, except to the
extent required by law.
S-14
Table of Contents
Use of proceeds
Based on the public offering price of $6.25 per share, we estimate that the net proceeds to us from this offering will be
approximately $108.0 million (or approximately $124.2 million if the underwriters exercise their option to purchase 2,760,000 additional shares in full), after deducting underwriting
discounts and commissions and estimated offering expenses payable by us.
We
intend to use the net proceeds from this offering to fund our research and development efforts, including clinical trials for our proprietary candidates, build and scale commercial capability, and
for general corporate purposes, including general working capital purposes. We may also use a portion of the net proceeds to acquire or invest in complementary businesses, technologies, drugs, drug
candidates or other intellectual property, although we have no present commitments or agreements to do so.
The
amounts and timing of these expenditures will depend on a number of factors, such as the timing and progress of our research and development efforts, technological advances and the competitive
environment for our drug candidates. As of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from this offering.
Accordingly, we will retain broad discretion over the use of these proceeds. Pending these uses, we intend to invest the net proceeds in investment-grade, interest-bearing securities.
S-15
Table of Contents
Dilution
If you invest in our common stock, your interest will be diluted to the extent of the difference between the public offering price
per share you pay in this offering and the net tangible
book value per share of our common stock immediately after this offering. Our net tangible book value as of June 30, 2016, was approximately $(37.9) million, or $(0.26) per share of common
stock. Net tangible book value per share is calculated by subtracting our total liabilities from our total tangible assets and dividing this amount by the number of shares of common stock outstanding
as of June 30, 2016. After giving effect to the sale by us of 18,400,000 shares of common stock in this offering at the public offering price of $6.25 per share and after deducting underwriting
discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of June 30, 2016 would have been approximately $70.0 million, or
approximately $0.43 per share of common stock. This represents an immediate increase in the net tangible book value of approximately $0.69 per share to our existing stockholders and an immediate and
substantial dilution in the net tangible book value of approximately $5.82 per share of common stock to investors purchasing our common stock in this offering. The following table illustrates this
calculation on a per share basis:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Public offering price per share
|
|
|
|
|
$
|
6.25
|
|
|
Net tangible book value per share as of June 30, 2016
|
|
$
|
(0.26
|
)
|
|
|
|
|
Increase in net tangible book value per share attributable to investors purchasing our common stock in this offering
|
|
$
|
0.69
|
|
|
|
|
|
As adjusted net tangible book value per share after this offering
|
|
|
|
|
$
|
0.43
|
|
|
Dilution per share to investors
|
|
|
|
|
$
|
5.82
|
|
|
|
|
|
|
|
|
|
|
The
information above assumes that the underwriters do not exercise their option to purchase additional shares. If the underwriters exercise their option to purchase additional shares in full, the as
adjusted net tangible book value as of June 30, 2016 would have been approximately $0.52 per share, representing an increase to existing stockholders of approximately $0.78 per share, and there
will be an immediate dilution of approximately $5.73 per share to investors purchasing our common stock in this offering at the public offering price.
The
above discussion and table are based on 143,690,104 shares of common stock outstanding as of June 30, 2016 and does not take into account:
-
•
-
11,647,595 shares of common stock issuable upon exercise of options outstanding as of June 30, 2016 at a weighted average
exercise price of $4.80 per share;
-
•
-
832,100 shares of common stock reserved for issuance upon vesting of restricted stock units as of June 30, 2016;
-
•
-
19,228,677 shares of common stock available for future issuance under our Amended and Restated Stock Option and Incentive Plan as of
June 30, 2016;
-
•
-
586,104 shares of common stock available for future issuance under our Amended and Restated Employee Stock Purchase Plan as of
June 30, 2016; and
-
•
-
18,761,527 shares of common stock issuable upon conversion of our 3.00% Convertible Senior Notes due in 2020.
S-16
Table of Contents
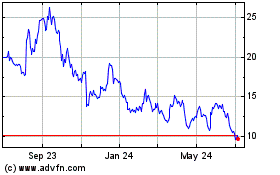
Price range of our common stock
Our common stock has been quoted on The NASDAQ Global Market under the symbol "ARRY" since our initial public offering on
November 17, 2000. The following table sets forth, for the periods indicated, the reported high and low sales prices per share of our common stock as reported by The NASDAQ Global Market:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal year ending June 30, 2017
|
|
High
|
|
Low
|
|
|
|
|
|
|
|
|
|
|
|
First Quarter (through September 27, 2016)
|
|
$
|
7.27
|
|
$
|
3.10
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal year ended June 30, 2016
|
|
High
|
|
Low
|
|
|
|
|
|
|
|
|
|
|
|
First Quarter
|
|
$
|
7.31
|
|
$
|
4.45
|
|
|
Second Quarter
|
|
$
|
5.57
|
|
$
|
3.72
|
|
|
Third Quarter
|
|
$
|
4.18
|
|
$
|
2.38
|
|
|
Fourth Quarter
|
|
$
|
4.29
|
|
$
|
2.60
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal year ended June 30, 2015
|
|
High
|
|
Low
|
|
|
|
|
|
|
|
|
|
|
|
First Quarter
|
|
$
|
4.81
|
|
$
|
3.51
|
|
|
Second Quarter
|
|
$
|
5.04
|
|
$
|
2.98
|
|
|
Third Quarter
|
|
$
|
8.59
|
|
$
|
4.19
|
|
|
Fourth Quarter
|
|
$
|
8.04
|
|
$
|
6.16
|
|
|
|
|
|
|
|
|
|
|
On
September 27, 2016, the closing price of our common stock as reported by The NASDAQ Global Market was $6.59 per share. As of September 26, 2016, there were approximately 54
stockholders of record of our common stock. This does not include the number of persons whose stock is held in nominee or "street name" accounts through brokers.
Dividend policy
We have never declared or paid any cash dividends on our common stock. We currently intend to retain any future earnings to finance
the growth and development of our business. Additionally, we are currently restricted from paying any dividends under our credit facilities. Therefore, we do not anticipate that we will declare or pay
any cash dividends on our common stock in the foreseeable future. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will depend on our financial
condition, results of operations, capital requirements, restrictions under any existing indebtedness and other factors the Board of Directors deems relevant.
S-17
Table of Contents
Material U.S. federal tax considerations to non-U.S. holders
The following is a general discussion of the principal material U.S. federal income and estate tax considerations with respect to the
acquisition, ownership and disposition of our common stock by a non-U.S. holder (as defined below) as of the date hereof. This discussion is not a complete analysis of all of the potential U.S.
federal income and estate tax consequences relating to the ownership of our stock, nor does it address any tax consequences arising under any state, local, or non-U.S. tax laws, the U.S. federal gift
tax rules or any other U.S. federal tax laws. Except where noted, this discussion deals only with a non-U.S. holder that holds our common stock as a capital asset within the meaning of
Section 1221 of the Internal Revenue Code of 1986, as amended, or the Code (generally, property held for investment).
For
purposes of this discussion, a "non-U.S. holder" means a beneficial owner of our common stock (other than an entity treated as a partnership) that is not any of the following for U.S. federal
income tax purposes: (i) an individual who is a citizen or resident of the U.S., (ii) a corporation (or other entity treated as a corporation for U.S. federal income tax purposes)
created or organized in or under the laws of the U.S., any state thereof, the District of Columbia, or any political subdivision of the United States, (iii) an estate the income of which is
subject to U.S. federal income taxation regardless of its source, or (iv) a trust if (1) its administration is subject to the primary supervision of a court within the U.S. and one or
more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) have the authority to control all of its substantial decisions, or (2) it has a valid election in effect under
applicable U.S. Treasury regulations to be treated as a U.S. person (within the meaning of Section 7701(a)(30) of the Code).
If
an entity classified as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner in the partnership will generally depend on the status of the
partner and upon the activities of the partnership. Accordingly, if you are a partnership holding our common stock, or a partner in such a partnership, you should consult your tax advisors regarding
the specific U.S. federal income tax consequences applicable to you.
This
discussion is based upon provisions of the Code, Treasury Regulations promulgated thereunder, rulings and judicial decisions as of the date hereof. Those authorities may be changed, perhaps
retroactively, or be subject to differing interpretations, so as to result in U.S. federal income and estate tax consequences different from those summarized below. No ruling has been or will be
sought from the Internal Revenue Service, or IRS, with respect to the matters discussed below, and there can be no assurance that the IRS will not take a contrary position regarding the tax
consequences of the acquisition, ownership or disposition of shares of our common stock, or that any such contrary position would not be sustained by a court. This discussion does not address all
aspects of U.S. federal income and estate taxes that may be relevant to non-U.S. holders in light of their personal circumstances or to non-U.S. holders who are subject
to special rules, including without limitation banks, thrifts or other financial institutions; insurance companies; partnerships, or other pass-through entities; real estate investment trusts;
regulated investment companies; former U.S. citizens or residents; "controlled foreign corporations" or "passive foreign investment companies"; corporations that accumulate earnings to avoid U.S.
federal income tax; brokers, dealers or traders in securities, commodities or currencies; tax-exempt organizations; tax-qualified retirement plans; persons subject to the alternative minimum tax;
persons that hold or receive shares of our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; persons that own, or are deemed to own, more than 5% of our
outstanding common stock (except to the extent specifically set forth below); persons holding shares of our common stock as part of a hedge, straddle or other risk reduction strategy or as part of a
conversion transaction or other integrated investment; and persons deemed to sell shares of our common stock under the constructive sale provisions
S-18
Table of Contents
of
the Code. In addition, this discussion does not address alternative minimum tax or the impact of the Medicare contribution tax on net investment income. We cannot assure you that a change in law
will not alter significantly the tax considerations that we describe in this discussion.
If you are considering the purchase of our common stock, you should consult your own tax advisors concerning the particular U.S. federal tax consequences to you of the
ownership and disposition of the common stock, as well as the consequences to you arising under the laws of any other taxing jurisdiction, including any state, local or foreign income tax
consequences.
Dividends
Payments made on our common stock will generally constitute "dividends" for U.S. federal income tax purposes to the extent paid from
our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return
of capital and will first be applied against and reduce a holder's adjusted tax basis in our common stock, but not below zero. Any excess amounts will be treated as gain from the sale of our common
stock and will be treated as described below under "—Sale or Other Taxable Disposition".
We
have never declared or paid cash dividends on our common stock and we do not intend to declare or pay cash dividends on our common stock in the foreseeable future. If we were to pay dividends in
the
future on our common stock, they would be subject to U.S. federal income tax in the manner described below.
Dividends
paid to a non-U.S. holder of our common stock generally will be subject to withholding of U.S. federal income tax at a 30% rate of the gross amount of the dividends or such lower rate as may
be specified by an applicable income tax treaty, provided the non-U.S. Holder furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the
lower treaty rate. However, dividends that are effectively connected with the conduct of a U.S. trade or business by a non-U.S. holder and, where an income tax treaty applies, are attributable to a
U.S. permanent establishment or fixed base of the non-U.S. holder, are not subject to this withholding tax, but instead are subject to U.S. federal income tax on a net income basis at, as the case may
be, generally applicable individual or corporate graduated rates. Certain certification and disclosure requirements must be complied with in order for effectively connected income to be exempt from
this withholding tax, including furnishing to us or our paying agent a properly completed and executed IRS Form W-8ECI (or successor form) certifying that the dividends are effectively
connected with the Non-U.S. Holder's conduct of a trade or business within the United States. Any such effectively connected dividends received by a foreign corporation may, under certain
circumstances, be subject to an additional "branch profits tax" at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. Non-U.S. Holders should consult their tax
advisors regarding any applicable tax treaties that may provide for different rules.
A
non-U.S. holder of our common stock who wishes to claim the benefit of an applicable treaty rate (and avoid backup withholding as discussed below) for dividends will be required to
(a) properly complete and execute IRS Form W-8BEN or W-8BEN-E (or successor form) and certify under penalty of perjury that such holder is not a U.S. person and is qualified for the
reduced rate or (b) if the common stock is held through certain foreign intermediaries or other agents acting on the holder's behalf, satisfy the relevant certification requirements of
applicable Treasury regulations. Non-U.S. holders must provide this certification to us or our paying agent prior to the payment of any dividends and it must be updated periodically. If the non-U.S.
holder holds the stock through a financial institution or other agent acting on the holder's behalf, the holder will be required to provide appropriate documentation to the agent. The
S-19
Table of Contents
holder's
agent will then be required to provide certification to us or our paying agent, either directly or through other intermediaries. These forms must be periodically updated. Non-U.S. holders
should consult their tax advisors regarding their entitlement to benefits under an applicable income tax treaty and the manner of claiming the benefits of such treaty.
A
non-U.S. holder of our common stock that does not timely provide us or our paying agent with the required certification, but that qualifies for a reduced treaty rate, may obtain a refund of any
excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
For
additional withholding rules that may apply to dividends paid to certain foreign entities, see the discussion below under the headings Foreign Account Tax Compliance Act and Information Reporting
and Backup Withholding.
Gain on disposition of common stock
Subject to the discussions below regarding the Foreign Account Tax Compliance Act and Information Reporting and Backup Withholding, a
non-U.S. holder generally will not be subject to U.S. federal income tax or withholding thereof with respect to gain recognized on a sale or other disposition of our common stock unless (i) the
gain is effectively connected with the non-U.S. holder's conduct of a trade or business in the U.S. and, where a tax treaty applies, is attributable to a U.S. permanent establishment or fixed base of
the non-U.S. holder, (in which case the net income basis taxation described above would apply, and, for a non-U.S. holder that is a foreign corporation, the branch profits tax described above may also
apply), (ii) the non-U.S. holder is a nonresident alien individual who holds our common stock as a capital asset, is present in the U.S. for 183 or more days during the taxable year of the sale
or other disposition and meets certain other requirements (in which case the gain would be subject to U.S. federal income tax at a flat 30% rate, or such lower rate as is specified by an applicable
tax treaty, but may be offset by U.S. source capital losses even though the individual is not considered a resident of the U.S., provided the non-U.S. holder has timely filed U.S. federal income tax
returns with respect to such losses), or (iii) we are or have been a "U.S. real property holding corporation," or USRPHC, for U.S. federal income tax purposes at any time during the shorter of
the five-year period ending on the date of disposition or the period that the non-U.S. holder held the common stock.
Generally,
a corporation is a USRPHC if the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests
plus its other assets used or held for use in a trade or business. We believe that we currently are not, and we do not anticipate becoming, a "USRPHC" for U.S. federal income tax purposes.. If we are
or become a USRPHC, and if our common stock is "regularly traded," as defined by applicable Treasury Regulations, on an established securities market at any time during the calendar year, only a
non-U.S. holder who holds or held, actually and constructively, (at any time during the shorter of the five-year period preceding the date of disposition or the holder's holding period) more than 5%
of our common stock will be subject to U.S. federal income tax on the disposition of the common stock. Such holder would be subject to regular U.S. federal income tax with respect to any gain
recognized in generally the same manner as a U.S. person.
Non-U.S.
Holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
S-20
Table of Contents
Federal estate tax
Common stock in a U.S. corporation, including Array, held by an individual who is a non-U.S. holder at the time of death will be
considered U.S. situs property, will be included in the gross estate of the nonresident alien decedent for U.S. federal estate tax purposes, and, therefore, may be subject to U.S. federal estate tax,
unless an applicable estate tax treaty provides otherwise.
Information reporting and backup withholding
Generally, we must report annually to the IRS and to each non-U.S. holder the amount of dividends paid to such holder and the tax
withheld (if any) with respect to such dividends, regardless of whether withholding was required. Copies of the information returns reporting such dividends and any withholding may also be made
available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of an applicable income tax treaty or agreement.
Under
certain circumstances, the Code imposes backup withholding, currently at a 28% rate, on certain reportable payments. Backup withholding, however, generally will not apply to payments of
dividends to a non-U.S. holder of our common stock if the non-U.S. holder furnishes to us or our paying agent the required certification under penalties of perjury as to its non-U.S. status, such as
by providing a valid IRS Form W-8BEN, W-8-BEN-E or W-8ECI, or certain other requirements are met. Notwithstanding the foregoing, backup withholding may apply if either we have or our paying
agent has actual knowledge, or reason to know, that the holder is a U.S. person that is not an exempt recipient.
Payments
of the proceeds from a disposition by a non-U.S. holder of our common stock made by or through a non-U.S. office of a non-U.S. broker generally will not be subject to information reporting or
backup withholding. However, information reporting (but not backup withholding) will apply to those payments if the broker does not have documentary evidence that the beneficial owner is a non-U.S.
holder, an exemption is not otherwise established, and the broker has certain connections with the United States.
Payment
of the proceeds from a non-U.S. holder's disposition of our common stock made by or through the U.S. office of a broker generally will be subject to information reporting and backup
withholding unless the non-U.S. holder certifies as to its non-U.S. holder status under penalties of perjury, such as by providing a valid IRS Form W-8BEN, W-8-BEN-E or W-8ECI, or otherwise
establishes an exemption from information reporting and backup withholding. Notwithstanding the foregoing, backup withholding may apply if either we have or our paying agent has actual knowledge, or
reason to know, that the holder is a U.S. person that is not an exempt recipient.
Backup
withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder's U.S. federal income tax
liability, provided the required information is timely furnished to the IRS.
Foreign account tax compliance act
Pursuant to Sections 1471 to 1474 of the Code and the Treasury regulations promulgated thereunder (FATCA), dividends paid in
respect of our common stock, and, after December 31, 2018, gross proceeds from the sale or other disposition of our common stock held by or through certain "foreign financial institutions" (as
specially defined under the Code for purposes of these rules, including investment funds) will be subject to withholding at a rate of 30%, unless (1) such institution enters into an agreement
with the Treasury to report, on an annual basis, information with respect to interests in, and accounts maintained by, the institution to the extent such interests or accounts are held by certain U.S.
persons and
S-21
Table of Contents
by
certain non-U.S. entities that are wholly or partially owned by U.S. persons and to withhold on certain payments or (2) such institution otherwise qualifies for an exemption from these
rules. An intergovernmental agreement between the U.S. and an applicable foreign country, or future Treasury regulations or other guidance, may modify these requirements. Accordingly, the entity
through which our common stock is held will affect the determination of whether such withholding is required. Similarly, dividends in respect of, and gross proceeds from the sale of, our common stock
held by an investor that is a "non-financial foreign entity" (as specially defined under the Code for purposes of these rules) will be subject to withholding at a rate of 30%, unless such entity
either (1) certifies to us that such entity does not have any "substantial United States owners" (as specifically defined under the Code for purposes of these rules) or provides certain
information regarding the entity's "substantial United States owners," which we will in turn provide to the IRS or (2) such non-financial foreign entity otherwise qualifies for an exemption
from these rules. We will not pay any additional amounts to non-U.S. holders in respect of any amounts withheld. A foreign financial institution or non-financial foreign entity can generally meet the
certification requirements by providing a properly executed IRS Form W-8BEN, IRS Form W-8BEN-E, or IRS Form W-8ECI, as applicable. Non-U.S. holders are encouraged to consult their
tax advisors regarding the possible implications of the legislation on their investment in our common stock.
THE PRECEDING DISCUSSION OF U.S. FEDERAL INCOME TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY AND IS NOT TAX ADVICE. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX
ADVISOR REGARDING THE PARTICULAR U.S. FEDERAL, STATE, LOCAL AND FOREIGN TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN
APPLICABLE LAWS.
S-22
Table of Contents
Underwriting
We are offering the shares of common stock described in this prospectus through a number of underwriters. J.P. Morgan
Securities LLC and Cowen and Company, LLC are acting as joint book-running managers of the offering and J.P. Morgan Securities LLC is acting as representative of the underwriters.
We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each
underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of shares of
common stock listed next to its name in the following table:
|
|
|
|
|
|
|
|
|
|
|
|
Name
|
|
Number of
shares
|
|
|
|
|
|
|
|
|
J.P. Morgan Securities LLC
|
|
|
9,200,000
|
|
|
Cowen and Company, LLC
|
|
|
4,600,000
|
|
|
Stifel, Nicolaus & Company, Incorporated
|
|
|
1,840,000
|
|
|
Wells Fargo Securities, LLC
|
|
|
1,840,000
|
|
|
SunTrust Robinson Humphrey, Inc.
|
|
|
920,000
|
|
|
|
|
|
|
|
|
Total
|
|
|
18,400,000
|
|
|
|
|
|
|
|
The
underwriters are committed to purchase all the common shares offered by us if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase
commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The
underwriters propose to offer the common shares directly to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers at that price less
a concession not in excess of $0.225 per share. After the initial offering of the shares to the public, the offering price and other selling terms may be changed by the underwriters. Sales of shares
made outside of the United States may be made by affiliates of the underwriters. The offering of the shares by the underwriters is subject to receipt and acceptance and is subject to the underwriters'
right to reject any order in whole or in part.
The
underwriters have an option to buy up to 2,760,000 additional shares of common stock from us to cover sales of shares by the underwriters which exceed the number of shares specified in the table
above. The underwriters have 30 days from the date of this prospectus to exercise this option to purchase additional shares. If any shares are purchased with this option to purchase additional
shares, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional shares of common stock are purchased, the underwriters will offer the
additional shares on the same terms as those on which the shares are being offered.
The
underwriting fee is equal to the public offering price per share of common stock less the amount paid by the underwriters to us per share of common stock. The underwriting fee is $0.375 per share.
The following table shows the per share and total public offering price, underwriting discounts and commissions
S-23
Table of Contents
to
be paid to the underwriters and proceeds before expenses to us assuming both no exercise and full exercise of the underwriters' option to purchase additional shares.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Per share
|
|
No exercise
|
|
Full exercise
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Public offering price
|
|
$
|
6.25
|
|
$
|
115,000,000
|
|
$
|
132,250,000
|
|
|
Underwriting discounts and commissions payable by us
|
|
$
|
0.375
|
|
$
|
6,900,000
|
|
$
|
7,935,000
|
|
|
Proceeds, before expenses, to us
|
|
$
|
5.875
|
|
$
|
108,100,000
|
|
$
|
124,315,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We
estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and
commissions, will be approximately $125,000. We have also agreed to reimburse the underwriters for certain expenses, including up to an aggregate of $25,000 in connection with the clearance of this
offering with the Financial Industry Regulatory Authority.
A
prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may
agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to
underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We
have agreed that we will not (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell,
grant any option, right or warrant to purchase or otherwise dispose of, directly or indirectly, or file with the Securities and Exchange Commission a registration statement under the Securities Act
relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer,
sale, pledge, disposition or filing, or (ii) enter into any swap or other arrangement that transfers all or a portion of the economic consequences associated with the ownership of any shares of
common stock or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of shares of common stock or such other securities, in cash or otherwise),
in each case without the prior written consent of J.P. Morgan Securities LLC for a period of 60 days after the date of this prospectus, other than the shares of our common stock to be
sold hereunder and any shares of our common stock issued upon the exercise of options granted under our existing stock-based compensation plans.
Our
directors and executive officers have entered into lock-up agreements with the underwriters prior to the commencement of this offering pursuant to which each of these persons, with limited
exceptions, for a period of 60 days after the date of this prospectus, may not, without the prior written consent of J.P. Morgan Securities LLC, (1) offer, pledge, announce
the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer
or dispose of, directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock (including, without limitation, common
stock or such other securities which may be deemed to be beneficially owned by such directors, executive officers, managers and members in accordance with the rules and regulations of the SEC and
securities which may be issued upon exercise of a stock option or warrant) or (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of
ownership of the common stock or such other securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of common stock or such other
securities, in cash or otherwise, or (3) make any demand for or exercise any right with respect to the registration of any shares
S-24
Table of Contents
of
our common stock or any security convertible into or exercisable or exchangeable for our common stock.
We
have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act of 1933.
Our
common stock is listed on The NASDAQ Global Market under the symbol "ARRY."
In
connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling shares of common stock in the open market for the
purpose of preventing or retarding a decline in the market price of the common stock while this offering is in progress. These stabilizing transactions may include making short sales of the common
stock, which involves the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open
market to cover positions created by short sales. Short sales may be "covered" shorts, which are short positions in an amount not greater than the underwriters' option to purchase additional shares
referred to above, or may be "naked" shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase
additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for
purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional shares. A naked short position is more likely to be created if
the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchase in this offering. To the
extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The
underwriters have advised us that, pursuant to Regulation M of the Securities Act of 1933, they may also engage in other activities that stabilize, maintain or otherwise affect the price of
the common stock, including the imposition of penalty bids. This means that if the representative of the underwriters purchase common stock in the open market in stabilizing transactions or to cover
short sales, the representative can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These
activities may have the effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price of the common stock, and, as a result, the
price of the common stock may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The
underwriters may carry out these transactions on The NASDAQ Global Market, in the over-the-counter market or otherwise.
In
addition, in connection with this offering certain of the underwriters (and selling group members) may engage in passive market making transactions in our common stock on The NASDAQ Global Market
prior to the pricing and completion of this offering. Passive market making consists of displaying bids on The NASDAQ Global Market no higher than the bid prices of independent market makers and
making purchases at prices no higher than these independent bids and effected in response to order flow. Net purchases by a passive market maker on each day are generally limited to a specified
percentage of the passive market maker's average daily trading volume in the common stock during a specified period and must be discontinued when such limit is reached. Passive market making may cause
the price of our common stock to be higher than the price that otherwise would exist in the open market in the absence of these transactions. If passive market making is commenced, it may be
discontinued at any time.
S-25
Table of Contents
The
underwriters and their respective affiliates are full-service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking,
financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Certain of the underwriters and their affiliates have provided in the
past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the
ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, in the ordinary course of their various business activities,
the underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans,
commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or
relate to our assets, securities and/or instruments (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with us. The underwriters and their
respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets,
securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
Selling restrictions
General
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by
this prospectus supplement in any jurisdiction where action for that purpose is required.
The
securities offered by this prospectus supplement may not be offered or sold, directly or indirectly, nor may this prospectus supplement or any other offering material or advertisements in
connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and
regulations of that jurisdiction. Persons into whose possession this prospectus supplement comes are advised to inform themselves about and to observe any restrictions relating to the offering and the
distribution of this prospectus supplement. This prospectus supplement does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus supplement in
any jurisdiction in which such an offer or a solicitation is unlawful.
Notice to prospective investors in Canada
The shares of common stock may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as
defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National
Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the common stock must be made in accordance with an exemption from, or in a transaction
not subject to, the prospectus requirements of applicable securities laws.
Securities
legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus supplement (including any amendment thereto)
contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser's
province or territory. The
S-26
Table of Contents
purchaser
should refer to any applicable provisions of the securities legislation of the purchaser's province or territory for particulars of these rights or consult with a legal advisor.
Pursuant
to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105
Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection
with this offering.
Notice to prospective investors in the European Economic Area
In relation to each Member State of the European Economic Area, each, a Relevant Member State, no offer of shares may be made to the public in that
Relevant Member State other than:
-
(a)
-
to
any legal entity which is a qualified investor as defined in the Prospectus Directive;
-
(b)
-
to
fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus
Directive, subject to obtaining the prior consent of the representative; or
-
(c)
-
in
any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided
that no such offer of shares shall require the Company or the representative to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus
pursuant to Article 16 of the Prospectus Directive.
Each
person in a Relevant Member State who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed that it is a "qualified investor"
within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive. In the case of any shares being offered to a financial intermediary as that
term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the
offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of
any shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representative has been
obtained to each such proposed offer or resale.
The
Company, the representative and their affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgements and agreements.
For
the purpose of the above provisions, the expression "an offer to the public" in relation to any shares in any Relevant Member State means the communication in any form and by any means of
sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in the Relevant
Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression "Prospectus Directive" means Directive 2003/71/EC, as amended, including by Directive
2010/73/EU.
Notice to prospective investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be
directed at persons who are "qualified investors" (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within
Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as
S-27
Table of Contents
amended,
or the Order, and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the
Order (all such persons together being referred to as "relevant persons"). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United
Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Notice to prospective investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or
regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of
Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in
Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither
this document nor any other offering or marketing material relating to the offering, the Company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In
particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of shares has not
been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes ("CISA"). The investor protection afforded to acquirers of interests in collective investment schemes under
the CISA does not extend to acquirers of shares.
Notice to prospective investors in the Dubai International Financial Centre
This prospectus supplement relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority, or
the DFSA. This prospectus supplement is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any
other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus supplement nor taken steps to verify
the information set forth herein and has no responsibility for the prospectus supplement. The shares to which this prospectus supplement relates may be illiquid and/or subject to restrictions on their
resale. Prospective purchasers of the shares offered should conduct their own due diligence on the shares. If you do not understand the contents of this prospectus supplement you should consult an
authorized financial advisor.
Notice to prospective investors in Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and
Investments Commission in relation to the offering. This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the
"Corporations Act"), and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any
offer in Australia of the shares may only be made to persons, or the Exempt Investors, who are "sophisticated investors" (within the meaning of section 708(8) of the Corporations Act),
"professional investors" (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or
S-28
Table of Contents
more
exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
The
shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in
circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise
or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This
prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any
securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs,
objectives and circumstances, and, if necessary, seek expert advice on those matters.
Notice to prospective investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to
"professional investors" as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in
the document being a "prospectus" as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No
advertisement, invitation or document relating to the shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or
elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than
with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" as defined in the Securities and Futures Ordinance and any
rules made under that Ordinance.
Notice to prospective investors in Japan
The shares have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended)
and, accordingly, will not be offered or sold, directly or indirectly, in Japan, or for the benefit of any Japanese Person or to others for re-offering or resale, directly or indirectly, in Japan or
to any Japanese Person, except in compliance with all applicable laws, regulations and ministerial guidelines promulgated by relevant Japanese governmental or regulatory authorities in effect at the
relevant time. For the purposes of this paragraph, "Japanese Person" shall mean any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Notice to prospective investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document
or material in connection with the offer or sale, or invitation for subscription or purchase, of Non-CIS Securities may not be circulated or distributed, nor may the Non-CIS Securities be offered or
sold, or be made the subject of an invitation for
subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act,
Chapter 289 of Singapore, or the SFA, (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions
specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
S-29
Table of Contents
Where
the Non-CIS Securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
-
(a)
-
a
corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the
entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
-
(b)
-
a
trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who
is an accredited investor,
securities
(as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries' rights and interest (howsoever described) in that trust shall not be transferred within six months
after that corporation or that trust has acquired the Non-CIS Securities pursuant to an offer made under Section 275 of the SFA except:
-
(a)
-
to
an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in
Section 275(1A) or Section 276(4)(i)(B) of the SFA;
-
(b)
-
where
no consideration is or will be given for the transfer;
-
(c)
-
where
the transfer is by operation of law;
-
(d)
-
as
specified in Section 276(7) of the SFA; or
-
(e)
-
as
specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore.
S-30
Table of Contents
Where you can find more information
We are subject to the information reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and
we file annual, quarterly and special reports, proxy statements and other information with the SEC relating to our business, financial results and other matters. The reports, proxy statements and
other information we file may be inspected and copied at prescribed rates at the SEC's Public Reference Room and via the SEC's website (see below for more information).
This
prospectus supplement and the accompanying prospectus are part of a registration statement on Form S-3 that we filed under the Securities Act with the SEC. This prospectus supplement and
the accompanying prospectus do not contain all of the information included in that registration statement and its accompanying exhibits and schedules. For further information with respect to our
securities and us, you should refer to that registration statement and its accompanying exhibits and schedules. Statements in this prospectus supplement and the accompanying prospectus concerning any
document that we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You
should review the complete document to evaluate these statements.
You
may inspect a copy of the registration statement of which this prospectus supplement is a part and its accompanying exhibits and schedules, as well as the reports, proxy statements and other
information we file with the SEC, without charge at the SEC's Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549, and you may obtain copies of all or any part of the
registration statement from those offices for a fee. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains a web site that
contains reports, proxy and information statements and other information regarding registrants that file electronically, including us. The address of the site is www.sec.gov. We maintain a website at
http://www.arraybiopharma.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus supplement or the accompanying prospectus, and
you should not consider it part of this prospectus supplement or the accompanying prospectus.
Incorporation of certain information by reference
The Securities and Exchange Commission, or the SEC, allows us to incorporate by reference the information that we file with the SEC,
which means that we can disclose important information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus supplement.
These documents may include periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as Proxy Statements. Any
documents that we subsequently file with the SEC will automatically update and replace the information previously filed with the SEC. Thus, for example, in the case of a conflict or inconsistency
between information set forth in this prospectus and information incorporated by reference into this prospectus, you should rely on the information contained in the document that was filed later.
This
prospectus supplement incorporates by reference the documents listed below that we have previously filed (under File No. 001-16633) with the SEC and any additional documents that we may
file with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act (which shall not include information furnished under Item 2.02 or Item 7.01 of any Current Report on
Form 8-K, including any financial statements or
exhibits relating thereto and furnished pursuant to Item 9.01) between the date of this prospectus and the termination of the offering of the securities. These documents contain important
information about us.
S-31
Table of Contents
-
•
-
Our Annual Report on Form 10-K for the fiscal year ended June 30, 2016 filed with the SEC August 19, 2016,
including information incorporated by reference from our Definitive Proxy Statement on Schedule 14A for our annual meeting of stockholders, filed with the SEC on September 12, 2016;
-
•
-
Our Current Reports on Form 8-K filed with the SEC on July 28, 2016, August 3, 2016, August 9, 2016,
August 30, 2016, September 1, 2016, September 2, 2016, September 14, 2016 and September 26, 2016; and
-
•
-
The description of our common stock contained in our Registration Statement on Form 8-A filed with the SEC on
November 16, 2000, and the description of our preferred stock purchase rights contained in our Registration Statement on Form 8-A filed with the SEC on August 3, 2001, including
any amendment or report filed for the purpose of updating such descriptions.
You
can obtain a copy of any or all of these documents, including any exhibits thereto, at no cost, by visiting the Investor Relations section of our web site at
http://www.arraybiopharma.com
or by
requesting them in writing or by telephone at the following address:
Array
BioPharma Inc.
3200 Walnut Street
Boulder, Colorado 80301
(303) 381-6600
Attention: Investor Relations
Statements
contained in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein referring to the contents of any contract or other document are not
necessarily complete. Where such contract or other document is listed as an exhibit to the Registration Statement on Form S-3, of which this prospectus supplement and the accompanying
prospectus form a part, or any document incorporated by reference therein, each such statement is qualified by the provisions in such exhibit, to which reference is hereby made.
Information
found on, or accessible through, our website or websites referenced in the documents incorporated by reference is not a part of, and is not incorporated into, this prospectus supplement or
the accompanying prospectus, and you should not consider it part of this prospectus supplement or the accompanying prospectus.
S-32
Table of Contents
Legal matters
The validity of the common stock offered by this prospectus supplement and the accompanying prospectus will be passed upon for us by
Gross Cutler Seiler Dupont, LLC, Boulder, Colorado. Latham & Watkins LLP, Costa Mesa, California, is counsel for the underwriters in connection with this offering.
Experts
The financial statements of Array BioPharma Inc. as of June 30, 2016 and 2015, and for each of the years in the
three-year period ended June 30, 2016, and management's assessment of the effectiveness of internal control over financial reporting as of June 30, 2016, have been incorporated by
reference herein and in the registration statement in reliance upon the reports of KPMG LLP, independent registered public accounting firm, incorporated by reference herein, and upon the
authority of said firm as experts in accounting and auditing.
S-33
Table of Contents
PROSPECTUS

ARRAY BIOPHARMA INC.
UP TO $300,000,000 OF OUR
COMMON STOCK
PREFERRED STOCK
DEPOSITARY SHARES
DEBT SECURITIES
WARRANTS
UNITS
We may offer from time to time up to $300,000,000 of any combination of the securities described in this prospectus, separately or together as
units or in separate series, in amounts, at prices and on terms to be set forth in one or more supplements to this prospectus at the time of an offering. Any preferred stock we sell may be sold as
either shares of preferred stock or represented by depositary shares.
Each
time we plan to issue securities, we will provide a prospectus supplement, which will contain a description of the securities being offered and information about the specific terms,
the public offering price of the securities and the net proceeds we expect to receive from such sale, and may add, update or change information contained in this prospectus. You should read this
prospectus and the prospectus supplements carefully before you invest.
The
securities may be sold directly by us to investors, through agents designated from time to time or to or through underwriters or dealers. We will set forth the names of any
underwriters or agents in an accompanying prospectus supplement. For additional information on the methods of sale, you should refer to the section entitled "Plan of Distribution."
Our
common stock is listed on the Nasdaq Global Market and traded under the symbol "ARRY." On August 17, 2016, the last reported sale price for our common stock was $3.45 per
share.
An investment in our securities involves a high degree of risk. You should carefully consider the "Risk Factors" contained in any supplements to
this prospectus and in our most recent annual report on Form 10-K and in our other filings made with the Securities and Exchange Commission, which are incorporated by reference in this
prospectus. See "Risk Factors" on page 2 of this prospectus.
This prospectus may not be used to offer or sell securities unless accompanied by a prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or
accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The
date of this prospectus is August 23, 2016
Table of Contents
TABLE OF CONTENTS
We
have not authorized anyone to provide you with any information other than information contained in or incorporated by reference in this prospectus, any prospectus supplement or any
free writing prospectus prepared by or on behalf of us or to which we have referred you. The information in this prospectus or any prospectus supplement is accurate only as of the date of the document
on the front of the document, and any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of
this prospectus or any sale of a security.
References
in this prospectus to "Array," "the company," "we," "our" or "us" refer to Array BioPharma Inc. Our trademarks include the Array BioPharma logo and the terms "ARRAY
BIOPHARMA". Other trademarks and trade names appearing in this prospectus are the property of the holders of such trademarks and trade names.
i
Table of Contents
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or the SEC, using a
"shelf" registration process. Under this shelf process, we may from time to time offer up to $300,000,000 in any combination of the securities described in this prospectus. We may sell these
securities either individually or as units consisting of one or more of such securities, each at prices, in amounts and on terms to be determined at the time of sale. The common stock, preferred
stock, depositary shares, debt securities, warrants and units are collectively referred to in this prospectus as the "securities." The securities offered pursuant to this prospectus may be one or more
series of issuances, and the total offering price of the securities will not exceed $300,000,000.
This
prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide a prospectus supplement that will contain specific
information about the terms of that offering. The prospectus supplement, or information incorporated by reference in this
prospectus or any prospectus supplement that is of a more recent date, may also add, update or change information contained in this prospectus. You should read both this prospectus and any prospectus
supplement together with the additional information described below under the heading "Where You Can Find More Information." These documents do not contain an offer to sell or solicitation of an offer
to buy the securities in any circumstance in which the offer or solicitation is unlawful. This prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus
supplement.
SPECIAL NOTE REGARDING
FORWARD-LOOKING STATEMENTS
This prospectus contains and incorporates by reference certain forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995. All statements that are not descriptions of historical facts are forward-looking statements, based on management's estimates, assumptions and projections that are
subject to risks and uncertainties. These statements can generally be identified by the use of forward-looking terminology such as "believes," "expects," "intends," "may," "will," "should," or
"anticipates" or similar terminology.
These
statements involve significant risks and uncertainties, including those discussed below and those described more fully in other reports filed by Array with the SEC. Because these
statements reflect our current expectations concerning future events, our actual results could differ materially from those anticipated in these forward-looking statements as a result of many factors.
These factors include, but are not limited to, our ability to continue to fund and successfully progress internal research and development efforts and to create effective, commercially viable drugs;
risks associated with our dependence on our collaborators for the clinical development and commercialization of our out-licensed drug candidates; the ability of our collaborators and of Array to meet
objectives tied to milestones and royalties; our ability to effectively and timely conduct clinical trials in light of increasing costs and difficulties in locating appropriate trial sites and in
enrolling patients who meet the criteria for certain clinical trials; risks associated with our dependence on third-party service providers to successfully conduct clinical trials within and outside
the United States; our ability to achieve and maintain profitability and maintain sufficient cash resources; the extent to which the pharmaceutical and biotechnology industries are willing to
in-license drug candidates for their product pipelines and to collaborate with and fund third parties on their drug discovery activities; our ability to out-license our proprietary candidates on
favorable terms; and our ability to attract and retain experienced scientists and management; and the risk factors set forth under the caption "Risk Factors" in our most recent annual report on
Form 10-K and our quarterly reports on Form 10-Q, and any amendments thereto we file with the SEC, and in any supplements to this prospectus. The forward-looking statements contained
herein represent our judgment as of the date of this prospectus. We undertake no duty or obligation to update any forward-looking statements to reflect the occurrence of events or circumstances after
the date of such statements or of anticipated or unanticipated events that alter any assumptions underlying such statements.
ii
Table of Contents
ABOUT ARRAY BIOPHARMA
Array BioPharma Inc. is a biopharmaceutical company focused on the discovery, development and commercialization of targeted
small molecule drugs to treat patients afflicted with cancer. Five registration studies are currently advancing. These programs include three cancer drugs, binimetinib (MEK162), encorafenib
(LGX818) and selumetinib (partnered with AstraZeneca).
Our
principal executive offices are located at 3200 Walnut Street, Boulder, Colorado 80301 and our phone number is (303) 381-6600. We were founded in 1998 and became a public
company in November 2000. We also maintain a web site at http://www.arraybiopharma.com, which provides additional information about our company and through which you can also access our SEC filings.
The information set forth on our web site is not part of this prospectus. Our stock is listed on the NASDAQ Global Market under the symbol "ARRY."
SUMMARY OF THE SECURITIES
We may offer any of the following securities, either individually or as units, with a total value of up to $300,000,000 from time to
time under this prospectus at prices and on terms to be determined at the time of the offering:
-
•
-
common stock;
-
•
-
preferred stock, in one or more series;
-
•
-
depositary shares;
-
•
-
debt securities, in one or more series;
-
•
-
warrants to purchase shares of common stock, shares of preferred stock, depositary shares or debt securities;
-
•
-
units comprised of one or more debt securities, shares of common stock, shares of preferred stock and warrants in any combination; or
-
•
-
any combination of the foregoing securities.
We
refer to our common stock, preferred stock, depositary shares, debt securities, warrants and units collectively in this prospectus as the "securities." This prospectus provides you
with a general description of the securities we may offer. Each time we offer a type or series of securities, we will provide a prospectus supplement that will describe the specific amounts, prices
and other important terms of the securities.
1
Table of Contents
RISK FACTORS
Investment in our securities involves risks. Prior to making a decision about investing in our securities, you should consider
carefully the risk factors, together with all of the other information contained or incorporated by reference in this prospectus and any prospectus supplement, including any risks described in the
section entitled "Risk Factors" contained in any supplements to this prospectus and in our most recent annual report on Form 10-K and in our quarterly reports on Form 10-Q filed with the
SEC, as well as any amendments thereto and in other filings we make with the SEC, which are incorporated herein by reference in their entirety. Each of these risk factors could adversely affect our
business, operating results and financial condition, which may result in the loss of all or part of your investment.
USE OF PROCEEDS
Except as described in any applicable prospectus supplement we have authorized for use in connection with a specific offering, we
currently intend to use the net proceeds from the sale of the securities offered by us hereunder, if any, to fund the costs of research and development activities and for general corporate purposes,
including working capital. The amounts and timing of our use of the net proceeds from the sale of securities under this prospectus will depend on a number of factors, such as the timing and progress
of our research and development efforts, the timing
and progress of any partnering and commercialization efforts and the competitive environment for our products. As of the date of this prospectus, we have no current plans, commitments or agreements
with respect to any particular use of any net proceeds and we cannot specify with certainty all of the particular uses for the net proceeds to us from the sale of the securities under this prospectus.
Accordingly, our management will have broad discretion in the timing and application of such proceeds.
2
Table of Contents
RATIO OF EARNINGS TO FIXED CHARGES
The following table sets forth our ratio of earnings to fixed charges for each of the periods indicated. You should read this table in
conjunction with the financial statements and notes to the financial statements that are incorporated by reference in this prospectus.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended June 30,
|
|
|
|
2016
|
|
2015
|
|
2014
|
|
2013
|
|
2012
|
|
|
Ratio of earnings to fixed charges
|
|
|
N/A
|
|
$
|
1.79
|
|
|
N/A
|
|
|
N/A
|
|
|
N/A
|
|
We
did not record earnings for any of the fiscal years in the five-year period ended June 30, 2016 other than for the year ended June 30, 2015. Accordingly, our earnings
were insufficient to cover fixed charges for such periods, and we are unable to disclose a ratio of earnings to fixed charges for such periods. We recorded earnings of $9.4 million for the
fiscal year ended June 30, 2015. The dollar amount of the deficiency in earnings available for fixed charges was $92.8 million, $85.3 million, $61.9 million,
$23.6 million, and $56.3 million, for the years ended June 30, 2016, 2014, 2013 and 2012, respectively. Our ratio of combined fixed charges and preference dividends to earnings
for any of the foregoing periods was equivalent to our ratio of earnings to fixed charges.
3
Table of Contents
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock and material provisions of our amended and restated certificate of incorporation and
bylaws is only a summary. The description is qualified in its entirety by the complete provisions of our amended and restated certificate of incorporation, which has been filed as an exhibit to the
registration statement on Form S-1 (file no. 333-45922) filed with the SEC on September 15, 2000, and the amendments thereto filed as exhibits to the current reports on
Form 8-K filed with the SEC on November 6, 2007 (File No. 001-16633), on October 29, 2012 (File No. 001-16633), and to the proxy statement filed as an appendix to
our proxy statement on Schedule 14A for the 2015 annual meeting of stockholders (File No. 001-1663), and our amended and restated bylaws, which have been filed as an exhibit to the
current report on Form 8-K filed with the SEC on November 4, 2008 (File No. 001-16633). Our amended and restated certificate of incorporation authorizes the issuance of up to
280,000,000 shares of common stock, par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share, of which our board of directors has designated 10,135 shares as
Series B Convertible Preferred Stock. As of August 15, 2016, 145,022,564 shares of common stock were issued and outstanding and no shares of Series B Convertible Preferred Stock
were issued and outstanding.
Listing
Our common stock is listed on the Nasdaq Global Market and traded under the symbol "ARRY."
Transfer Agent and Registrar
American Stock Transfer and Trust Company is our transfer agent and registrar.
Common Stock
Each holder of common stock is entitled to one vote for each share on all matters to be voted upon by the stockholders. Holders of
common stock are not entitled to cumulative voting rights with respect to the election of directors. Subject to preferences that may be applicable to any preferred stock outstanding at the time,
holders of common stock are entitled to receive ratable dividends, if any, as may be declared from time to time by the board of directors out of funds legally available for that purpose. In the event
of our liquidation, dissolution or winding up, holders of common stock would be entitled to share ratably in all assets remaining after the payment of liabilities and liquidation preferences on any
outstanding preferred stock. Holders of common stock have no preemptive or conversion rights or other subscription rights and there are no redemption or sinking fund provisions applicable to the
common stock.
Preferred Stock
Our board of directors is authorized, without stockholder approval, to issue up to an aggregate of 10,000,000 shares of preferred stock
in one or more series. To date, our
board of directors has designated 10,135 shares of preferred stock as Series B Convertible Preferred Stock, the rights, preferences, privileges and restrictions of which are set forth in the
certificate of designation for the Series B Convertible Preferred Stock, which we filed with the SEC on May 3, 2011 as an exhibit to a current report on Form 8-K. All 10,135
shares of Series B Convertible Preferred Stock that were previously issued and were outstanding have been converted into 10,135,000 shares of common stock pursuant to the terms of the
Series B Convertible Preferred Stock, and no shares of preferred stock are currently outstanding. The board of directors can fix the rights, preferences, privileges and restrictions of any
other designated series of preferred stock, including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption, redemption prices, liquidation preferences and the number
of
4
Table of Contents
shares
constituting any series, or the designation of such series, without further vote or action by the stockholders.
The
rights, preferences, privileges and restrictions of any series of preferred stock will be set forth in a certificate of designation relating to that series. We will file as exhibits
to the registration statement of which this prospectus is a part, or will incorporate by reference from reports that we file with the SEC, the form of any certificate of designation that describes the
terms of the series of preferred stock we are offering before the issuance of the related series of preferred stock. We urge you to read the complete certificate of designation containing the terms of
the applicable series of preferred stock, as well as the applicable prospectus supplement related to such series. The certificate of designation will
include:
-
•
-
the title and stated value; the number of shares we are offering;
-
•
-
the liquidation preference per share;
-
•
-
the purchase price;
-
•
-
the dividend rate, period and payment date and method of calculation for dividends;
-
•
-
whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
-
•
-
the procedures for any auction and remarketing, if any;
-
•
-
the provisions for a sinking fund, if any;
-
•
-
the provisions for redemption or repurchase, if applicable, and any restrictions on our ability to exercise those redemption and
repurchase rights;
-
•
-
any listing of the preferred stock on any securities exchange or market;
-
•
-
whether the preferred stock will be convertible into our common stock, and, if applicable, the conversion price, or how it will be
calculated, and the conversion period;
-
•
-
voting rights, if any, of the preferred stock;
-
•
-
preemption rights, if any;
-
•
-
restrictions on transfer, sale or other assignment, if any;
-
•
-
whether interests in the preferred stock will be represented by depositary shares;
-
•
-
a discussion of any material or special United States federal income tax considerations applicable to the preferred stock;
-
•
-
the relative ranking and preferences of the preferred stock as to dividend rights and rights if we liquidate, dissolve or wind up our
affairs;
-
•
-
any limitations on issuance of any class or series of preferred stock ranking senior to or on a parity with the series of preferred
stock as to dividend rights and rights if we liquidate, dissolve or wind up our affairs; and
-
•
-
any other specific terms, preferences, rights or limitations of, or restrictions on, the preferred stock.
If
we issue shares of preferred stock under this prospectus, the shares will be fully paid and non-assessable.
The
General Corporation Law of the State of Delaware, the state of our incorporation, provides that the holders of preferred stock will have the right to vote separately as a class on
any proposed
5
Table of Contents
fundamental
change in the rights of the preferred stock. This right is in addition to any voting rights that may be provided for in the applicable certificate of designation.
We
may amend from time to time our amended and restated certificate of incorporation to increase the number of authorized shares of preferred stock. Any such amendment would require the
approval of the holders of a majority of the voting power of the shares entitled to vote thereon.
Future
issuances of preferred stock may have the effect of delaying or preventing a change in our control or make removal of our management more difficult. Additionally, the issuance of
preferred stock could decrease the amount of earnings and assets available for distribution to the holders of the common stock or could adversely affect the rights and powers, including voting rights,
of the holders of our common stock. The issuance of preferred stock could also cause the market price of our common stock to decline.
Registration Rights
Prior to our initial public offering and in connection with the sale and issuance of our Series A preferred stock in May 1998,
and August 1998, our Series B preferred stock in November 1999, and our Series C preferred stock in August 2000 (all of which shares converted into shares of our common stock in
connection with our initial public offering), we entered into an agreement with the investors in such financings providing for registration rights with respect to the shares of common stock, including
those issuable upon conversion of each series of preferred stock, held and subsequently acquired by these investors. Currently, 1.6 million shares of our common stock are entitled to
registration rights pursuant to terms and conditions of this agreement. The registration rights under this agreement allow the holders of at least 30% of the shares of common stock held by such
holders then outstanding to require us to register their shares under the Securities Act of 1933, as
amended, or the Securities Act, on up to two occasions, subject to limitations described in the agreement. In addition, these holders can require us to include their shares in future registrations of
our shares for our account or the account of another stockholder. These holders may also require us to register their shares on up to two occasions in any calendar year on Form S-3. These
registration rights are subject to limitations and conditions, including the right of underwriters to limit the number of shares of common stock held by existing stockholders to be included in a
registration. The registration rights as to any holder will terminate when all securities held by the holder entitled to registration rights can be sold within a three-month period under
Rule 144 of the Securities Act and when the number of shares held by the holder is less than 1% of our outstanding capital stock on an as converted to common stock basis. In addition, we are
generally required to bear all expenses of registration, including the reasonable fees of a single counsel acting on behalf of all selling stockholders, except underwriting discounts and selling
commissions.
In
connection with Facility Agreements we entered into with Deerfield Private Design Fund, L.P. and Deerfield Private Design International, L.P., healthcare investment
funds, who we collectively refer to as the Deerfield Funds, we issued warrants to purchase an aggregate of 12,000,000 shares of our common stock to the Deerfield Funds and entered into a Registration
Rights Agreement dated May 15, 2009. Under the terms of the Registration Rights Agreement, we agreed to file a registration statement with the SEC to register the resale of the shares of
the common stock subject to issuance upon the exercise of the warrants under the Securities Act. On August 31, 2009, we filed a registration statement on Form S-3 (File
No. 333-161633), which we amended on Forms S-3/A filed on September 24, 2009 and on September 30, 2009, registering the shares issuable upon exercise of the warrants, and
which the SEC declared effective as of October 9, 2009. We are generally required to bear all expenses of registration, including the reasonable fees of a single counsel acting on behalf of all
selling stockholders, except underwriting discounts and selling commissions. We have issued approximately 235,000 shares of common stock upon exercise of the warrants issued to the Deerfield Funds.
The remaining warrants expired on June 30, 2016.
6
Table of Contents
Registration
of any shares with registration rights would result in those shares becoming freely tradeable without restriction under the Securities Act. Sales of these shares, whether
pursuant to Rule 144 under the Securities Act or an effective registration statement, could have a material adverse effect on the trading price of our common stock.
Limitation of Liability of Directors
As permitted by the Delaware General Corporation Law, our amended and restated certificate of incorporation provides that our directors
are not personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director, except for liability:
-
•
-
for any breach of the director's duty of loyalty to us or our stockholders;
-
•
-
for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
-
•
-
under Section 174 of the Delaware General Corporation Law, relating to unlawful dividends or unlawful stock purchases or
redemptions; or
-
•
-
for any transaction from which the director derives an improper personal benefit.
As
a result of this provision, we and our stockholders may be unable to obtain monetary damages from a director for breach of his or her duty of care.
Indemnification
Our bylaws provide for the indemnification of our directors and officers to the fullest extent authorized by the Delaware General
Corporation Law. We will indemnify a director or officer in connection with an action initiated by that person if the action was authorized by our board of directors. The indemnification provided
under our bylaws includes the right to be paid expenses in advance of the final disposition of a proceeding for which indemnification may be had if the director or officer agrees to repay all amounts
paid in advance if it is ultimately determined that the director or officer is not entitled to be indemnified. Under our bylaws, if we do not pay a claim for indemnification within 60 days
after we have received a written claim, the director or officer may bring an action to recover the unpaid amount of the claim. If successful, the director or officer also will be entitled to be paid
the expense of prosecuting the action to recover these unpaid amounts.
Our
bylaws also authorize us to purchase and maintain insurance on behalf of any person who is or was one of our directors, officers, employees or agents, or is or was serving at our
request as a director, officer, employee, partner or agent of another corporation or other entity or enterprise, against any liability asserted against the person or incurred by the person in any of
these capacities, or arising out of the person's fulfilling one of these capacities, and related expenses. We may obtain this insurance whether or not we would have the power to indemnify the person
against the claim under the provisions of the Delaware General Corporation Law. We have purchased director and officer liability insurance on behalf of our directors and officers. The indemnification
provisions under our amended and restated certificate of incorporation and bylaws are not exclusive of any other rights to indemnification under any bylaw, agreement, vote of stockholders or
disinterested directors or otherwise.
Anti-Takeover Provisions
Our amended and restated certificate of incorporation and bylaws contain some provisions that are intended to enhance the likelihood of
continuity and stability in the composition of our board of directors and in the policies formulated by our board of directors. In addition, provisions of the
7
Table of Contents
Delaware
General Corporation Law may hinder or delay an attempted takeover of us other than through negotiation with our board of directors. These provisions could have the effect of discouraging
attempts to acquire us or remove incumbent management even if some or a majority of our stockholders believe this action is in their best interest, including attempts that might result in the
stockholders receiving a premium over the market price for the shares of common stock they hold.
Our amended and restated certificate of incorporation provides for the division of our board of directors into three groups of
directors serving staggered three-year terms. Our amended and restated certificate of incorporation further provides that the approval of the holders of at least two-thirds of the shares entitled to
vote is necessary for the alteration, amendment or repeal of sections of our amended and restated certificate of incorporation relating to the election and staggering of our board of directors,
limitation of director liability, indemnification and the vote requirements for these amendments to our amended and restated certificate of incorporation. These provisions may have the effect of
deterring hostile takeovers or delaying changes in control or management.
Our amended and restated certificate of incorporation provides that directors may be removed only with cause upon the affirmative vote
of holders representing two-thirds of our outstanding shares. In addition, vacancies and newly created directorships resulting from any increase in the size of the board of directors may be filled
only by the affirmative vote of a majority of the directors then in office, even if they do not constitute a quorum, or by the sole remaining director. These provisions would prevent stockholders from
removing incumbent directors without cause and filling the resulting vacancies with their own nominees.
Our bylaws establish an advance notice procedure with regard to the nomination, other than by the board of directors, of candidates for
election to the board of directors and with regard to matters to be brought before an annual meeting of our stockholders by a stockholder. The stockholder's notice must contain specified information
regarding the stockholder and its holdings, as well as about the director nominee and any business desired to be brought before the meeting. Although our bylaws do not give our board of directors any
power to approve or disapprove stockholder nominations for the election of directors or any other business desired by stockholders to be conducted at an annual meeting, the
bylaws:
-
•
-
may have the effect of precluding a nomination for the election of directors or precluding the conduct of business at a particular
annual meeting if the proper procedures are not followed; or
-
•
-
may discourage or deter a third party from conducting a solicitation of proxies to elect its own slate of directors or otherwise
attempting to obtain control of us, even if the conduct of this solicitation or the attempt to obtain control might be beneficial to us and our stockholders.
Under our amended and restated certificate of incorporation and bylaws, special meetings of stockholders, unless otherwise prescribed
by statute, may be called only by the board of directors, the chairperson, or the chief executive officer.
8
Table of Contents
Our amended and restated certificate of incorporation provides that any action required or permitted to be taken at a stockholders'
meeting may be taken without a meeting only by unanimous written consent.
Under Section 203 of the Delaware General Corporation Law, we may not engage in a "business combination," which includes a
merger or sale of more than 10% of our assets, with any "interested stockholder," namely, a stockholder who owns 15% or more of our outstanding voting stock, as well as affiliates and associates of
any of these persons, for three years following the time that stockholder became an interested stockholder, unless:
-
•
-
the transaction in which the stockholder became an interested stockholder is approved by our board of directors prior to the time the
interested stockholder attained that status;
-
•
-
upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder
owned at least 85% of our voting stock outstanding at the time the transaction commenced, excluding those shares owned by persons who are directors and also officers; or
-
•
-
at or after the time the stockholder became an interested stockholder the business combination is approved by the board of directors
and authorized at an annual or special meeting of stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock that is not owned by the interested stockholder.
The authorization of undesignated preferred stock makes it possible for the board of directors to issue preferred stock with voting or
other rights or preferences that could impede the success of any attempt to change control of our company. These and other provisions may have the effect of deterring hostile takeovers or delaying
changes in control or management of our company.
9
Table of Contents
DESCRIPTION OF DEPOSITARY SHARES
We may offer fractional shares of preferred stock rather than full shares of preferred stock, and, in that event, will issue receipts
for depositary shares. Each of these depositary shares will represent a fraction, which will be set forth in the applicable prospectus supplement, of a share of the applicable series of preferred
stock.
The
shares of any series of preferred stock underlying any depositary shares that we may sell under this prospectus will be deposited under a deposit agreement between us and a
depositary selected by us. Subject to the terms of the deposit agreement, each holder of a depositary share will be entitled, in proportion to the applicable fraction of a share of the preferred stock
underlying the depositary share, to all of the rights, preferences and privileges, and be subject to the qualifications and restrictions, of the preferred stock underlying that depositary share.
The
depositary shares will be evidenced by depositary receipts issued under a deposit agreement. Depositary receipts will be distributed to the holders of the depositary shares that are
sold in the applicable offering. We will incorporate by reference into the registration statement of which this prospectus is a part the form of any deposit agreement, including a form of depositary
receipt, that describes the terms of any depositary shares we are offering before the issuance of the related depositary shares. The following summaries of material provisions of the deposit
agreement, the depositary shares and the depositary receipts are subject to, and qualified in their entirety by reference to, all of the provisions of the deposit agreement applicable to a particular
offering of depositary shares. We urge you to read the applicable prospectus supplement relating to any depositary shares that are sold under this prospectus, as well as the complete deposit agreement
and depositary receipt.
Form
Pending the preparation of definitive depositary receipts, the depositary may, upon our written order, issue temporary depositary
receipts substantially identical to the definitive depositary receipts but not in definitive form. These temporary depositary receipts entitle their holders to all of the rights of definitive
depositary receipts. Temporary depositary receipts will then be exchangeable for definitive depositary receipts at our expense.
Dividends and Other Distributions
The depositary will distribute all cash dividends or other cash distributions received with respect to the underlying preferred stock
to the record holders of depositary shares in proportion to the number of depositary shares owned by those holders.
If
there is a distribution other than in cash, the depositary will distribute property received by it to the record holders of depositary shares in proportion to the number of depositary
shares owned by those holders, unless the depositary determines that it is not feasible to do so. If this occurs, the depositary may, with our approval, sell the property and distribute the net
proceeds from the sale to those holders in proportion to the number of depositary shares owned by them.
Withdrawal of Underlying Preferred Stock
Except as otherwise provided in a prospectus supplement, holders may surrender depositary receipts at the principal office of the
depositary and, upon payment of any unpaid amount due to the depositary, be entitled to receive the number of whole shares of underlying preferred stock and all money and other property represented by
the related depositary shares. We will not issue any partial shares of preferred stock. If the holder delivers depositary receipts evidencing a number of depositary shares that represent more than a
whole number of shares of preferred stock, the depositary will issue a new depositary receipt evidencing the excess number of depositary shares to the holder.
10
Table of Contents
Redemption of Depositary Shares
If the preferred stock underlying any depositary shares we may sell under this prospectus is subject to redemption, the depositary
shares will be redeemed from the proceeds received by the depositary resulting from any such redemption, in whole or in part, of that underlying preferred stock. The redemption price per depositary
share will be equal to the applicable fraction of the redemption price per share payable with respect to the underlying preferred stock. Whenever we redeem shares of underlying preferred stock that
are held by the depositary, the depositary will redeem, as of the same redemption date, the number of depositary shares representing the shares of underlying preferred stock so redeemed. If fewer than
all of the depositary shares are to be redeemed, the depositary shares to be redeemed will be selected by lot or proportionately, as may be determined by the depositary.
Voting
Upon receipt of notice of any meeting at which holders of the preferred stock underlying any depositary shares that we may sell under
this prospectus are entitled to vote, the depositary will mail the information contained in the notice to the record holders of the depositary
shares. Each record holder of the depositary shares on the record date, which will be the same date as the record date for the underlying preferred stock, will be entitled to instruct the depositary
as to the exercise of the voting rights pertaining to the amount of the underlying preferred stock represented by the holder's depositary shares. The depositary will then try, as far as practicable,
to vote the number of shares of preferred stock underlying those depositary shares in accordance with those instructions, and we will agree to take all reasonable actions which may be deemed necessary
by the depositary to enable the depositary to do so. The depositary will not vote the underlying preferred stock to the extent it does not receive specific instructions with respect to the depositary
shares representing such preferred stock.
Conversion of Preferred Stock
If the prospectus supplement relating to any depositary shares that we may sell under this prospectus states that the underlying
preferred stock is convertible into our common stock or other securities, the following will apply. The depositary shares, as such, will not be convertible into any of our securities. Rather, any
holder of the depositary shares may surrender the related depositary receipts to the depositary with written instructions that direct us to cause conversion of the preferred stock represented by the
depositary shares into or for whole shares of our common stock or other securities, as applicable. Upon receipt of those instructions and any amounts payable by the holder in connection with the
conversion, we will cause the conversion using the same procedures as those provided for conversion of the underlying preferred stock. If only some of a holder's depositary shares are converted, a new
depositary receipt or receipts will be issued to the holder for any depositary shares not converted.
Amendment and Termination of the Deposit Agreement
The form of depositary receipt evidencing the depositary shares and any provision of the deposit agreement may at any time be amended
by agreement between us and the depositary. However, any amendment which materially and adversely alters the rights of the holders of depositary shares will not be effective until 90 days after
notice of that amendment has been given to the holders. Each holder of depositary shares at the time any amendment becomes effective shall be deemed to consent and agree to that amendment and to be
bound by the deposit agreement as so amended. The deposit agreement may be terminated by us or by the depositary only if all outstanding depositary shares have been redeemed or converted into any
other securities into which the underlying preferred stock is convertible or there has been a final distribution, including to holders of depositary receipts, of the underlying preferred stock in
connection with our liquidation, dissolution or winding up.
11
Table of Contents
Charges of Depositary
We will pay all charges of the depositary, except for taxes and governmental charges and other charges as are expressly provided for in
the deposit agreement to be for the account of the holders of depositary shares or persons other than ourselves who may deposit any underlying preferred stock with the depositary.
Reports
The depositary will forward to holders of depositary receipts all notices and reports from us that we deliver to the depositary and
that we are required to furnish to the holders of the underlying preferred stock.
Limitation on Liability
Neither we nor the depositary will be liable if either of us is prevented or delayed by law or any circumstance beyond our control in
performing our respective obligations under the deposit agreement. Our obligations and those of the depositary will be limited to performance of our respective duties under the deposit agreement
without, in our case, negligence or bad faith or, in the case of the depositary, negligence or willful misconduct. We and the depositary may rely upon advice of counsel or accountants, or upon
information provided by persons presenting the underlying preferred stock for deposit, holders of depositary receipts or other persons believed by us in good faith to be competent and on documents
believed to be genuine.
Resignation and Removal of Depositary
The depositary may resign at any time by delivering notice to us of its election to resign. We may remove the depositary at any time.
Any resignation or removal will take effect upon the appointment of a successor depositary and its acceptance of the appointment. The successor depositary must be appointed within 60 days after
delivery of the notice of resignation or removal and must be a bank or trust company having its principal office in the United States and having a combined capital and surplus of at least $50,000,000.
DESCRIPTION OF DEBT SECURITIES
We may issue debt securities from time to time, in one or more series, as either senior or subordinated debt or as senior or
subordinated convertible or exchangeable debt. The particular terms of any series of debt securities and the extent to which the general provisions described herein may apply to a particular series of
debt securities will be described in the prospectus supplement relating to that series.
We
will issue any debt securities under an indenture between us and Wells Fargo Bank, National Association, as trustee thereunder (the "trustee"). The following summary of material
provisions of such debt securities and the indenture is subject to, and qualified in its entirety by reference to, all of the provisions of the supplemental indenture applicable to a particular series
of debt securities. We urge you to read the applicable prospectus supplement related to the debt securities that we may offer under this prospectus, as well as the complete indenture that contains the
terms of such debt securities.
General
The indenture does not limit the amount of debt securities that we may issue. The indenture provides that we may issue debt securities
up to the principal amount that we may authorize, which may be in any currency or currency unit that we may designate. Except for the limitations on consolidation, merger and sale of all or
substantially all of our assets contained in the indenture, the
12
Table of Contents
terms
of the indenture do not contain any covenants or other provisions designed to give holders of any debt securities protection against changes in our operations, financial condition or
transactions involving us. For each series of debt securities, any restrictive covenants for those debt securities will be described in the applicable prospectus supplement for those debt securities.
We
may issue the debt securities issued under the indenture as "discount securities," which means they may be sold at a discount below their stated principal amount. These debt
securities, as well as other debt securities that are not issued at a discount, may, for United States federal income tax purposes, be treated as if they were issued with "original issue discount," or
OID, because of interest payment and other characteristics. Special United States federal income tax considerations applicable to debt securities issued with original issue discount will be described
in more detail in any applicable prospectus supplement.
You
should refer to the prospectus supplement relating to a particular series of debt securities for a description of the following terms of the debt securities offered by that
prospectus supplement and by this prospectus:
-
•
-
the title and authorized denominations of those debt securities;
-
•
-
any limit on the aggregate principal amount of that series of debt securities;
-
•
-
the date or dates on which principal and premium, if any, of the debt securities of that series is payable;
-
•
-
interest rates, and the dates from which interest, if any, on the debt securities of that series will accrue, and the dates when
interest is payable and the maturity;
-
•
-
the right, if any, to extend the interest payment periods and the duration of the extensions;
-
•
-
the applicability of any guarantees;
-
•
-
if the amount of payments of principal or interest is to be determined by reference to an index or formula, or based on a coin or
currency other than that in which the debt securities are stated to be payable, the manner in which these amounts are determined and the calculation agent, if any, with respect thereto;
-
•
-
the place or places where and the manner in which principal of, premium, if any, and interest, if any, on the debt securities of that
series will be payable and the place or places where those debt securities may be presented for transfer and, if applicable, conversion or exchange;
-
•
-
the period or periods within which, the price or prices at which, the currency or currencies in which, and other terms and conditions
upon which those debt securities may be redeemed, in whole or in part, at our option or the option of a holder of those securities, if we or a holder is to have that option;
-
•
-
our obligation or right, if any, to redeem, repay or purchase those debt securities pursuant to any sinking fund or analogous
provision or at the option of a holder of those securities, and the terms and conditions upon which the debt securities will be redeemed, repaid or purchased, in whole or in part, pursuant to that
obligation;
-
•
-
the terms, if any, on which the debt securities of that series and any guarantees thereof will be subordinate in right and priority of
payment to our other debt;
-
•
-
the denominations in which those debt securities will be issuable;
-
•
-
if other than the entire principal amount of the debt securities when issued, the portion of the principal amount payable upon
acceleration of maturity as a result of a default on our obligations;
13
Table of Contents
-
•
-
whether those debt securities will be issued in fully registered form without coupons or in a form registered as to principal only
with coupons or in bearer form with coupons;
-
•
-
whether any securities of that series are to be issued in whole or in part in the form of one or more global securities and the
depositary for those global securities;
-
•
-
if other than United States dollars, the currency or currencies in which payment of principal of or any premium or interest on those
debt securities will be payable;
-
•
-
if the principal of or any premium or interest on the debt securities of that series is to be payable, or is to be payable at our
election or the election of a holder of those securities, in securities or other property, the type and amount of those securities or other property, or the manner of determining that amount, and the
period or periods within which, and the terms and conditions upon which, any such election may be made;
-
•
-
the events of default and covenants relating to the debt securities that are in addition to, modify or delete those described in this
prospectus;
-
•
-
conversion or exchange provisions, if any, including conversion or exchange prices or rates and adjustments thereto;
-
•
-
whether and upon what terms the debt securities may be defeased, if different from the provisions set forth in the indenture;
-
•
-
the nature and terms of any security for any secured debt securities;
-
•
-
the terms applicable to any debt securities issued at a discount from their stated principal amount; and
-
•
-
any other specific terms of any debt securities.
The
applicable prospectus supplement will present material United States federal income tax considerations for holders of any debt securities and the securities exchange or quotation
system on which any debt securities are to be listed or quoted.
Conversion or Exchange Rights
Debt securities may be convertible into or exchangeable for shares of our capital stock or other securities. The terms and conditions
of conversion or exchange will be stated in the applicable prospectus supplement. The terms will include, among others, the following:
-
•
-
the conversion or exchange price;
-
•
-
the conversion or exchange period;
-
•
-
provisions regarding our ability or the ability of any holder to convert or exchange the debt securities;
-
•
-
events requiring adjustment to the conversion or exchange price; and
-
•
-
provisions affecting conversion or exchange in the event of our redemption of the debt securities.
Consolidation, Merger or Sale
The terms of the indenture prevent us from consolidating or merging with or into, or conveying, transferring or leasing all or
substantially all of our assets to, any person, unless (i) we are the surviving corporation or the successor corporation or person to which our assets are conveyed, transferred or leased is
organized under the laws of the United States, any state of the United States or the District
14
Table of Contents
of
Columbia and it expressly assumes our obligations under the debt securities and the indenture and (ii) immediately after completing such a transaction, no event of default under the
indenture, and no event that, after notice or lapse of time or both, would become an event of default under the indenture, has occurred and is continuing. When the person to whom our assets are
conveyed or transferred has assumed our obligations under the debt securities and the indenture, we will be discharged from all our obligations under the debt securities and the indenture except in
limited circumstances.
This
covenant would not apply to any recapitalization transaction, a change of control affecting us or a highly leveraged transaction, unless the transaction or change of control were
structured to include a merger or consolidation or conveyance, transfer or lease of all or substantially all of our assets.
Events of Default
The indenture provides that the following will be "events of default" with respect to any series of debt
securities:
-
•
-
failure to pay interest for 30 days after the date payment is due and payable;
-
•
-
failure to pay principal or premium, if any, on any debt security when due, either at maturity, upon any redemption, by declaration or
otherwise;
-
•
-
failure to make sinking fund payments when due and continuance of such default for a period of 30 days;
-
•
-
failure to perform other covenants for 60 days after notice of such default or breach and request for it to be remedied;
-
•
-
specified events of bankruptcy, insolvency or reorganization relating to us; or
-
•
-
any other event of default provided in the applicable officer's certificate, resolution of our board of directors or the supplemental
indenture under which we issue a series of debt securities.
An
event of default for a particular series of debt securities does not necessarily constitute an event of default for any other series of debt securities issued under the indenture. For
each series of debt securities, any modifications to the above events of default will be described in the applicable prospectus supplement for those debt securities.
The
indenture provides that if an event of default specified in the first, second, third, fourth or sixth bullets above occurs and is continuing, either the trustee or the holders of at
least 25% in aggregate principal amount of the outstanding debt securities of that series may declare the principal amount of all those debt securities (or, in the case of discount securities or
indexed securities, that portion of the principal amount as may be specified in the terms of that series) to be due and payable immediately. If an event of default specified in the fifth bullet above
occurs and is continuing, then the principal amount of all those debt securities (or, in the case of discount securities or indexed securities, that portion of the principal amount as may be specified
in the terms of that series) will be due and payable immediately, without any declaration or other act on the part of the trustee or any holder. In certain cases, the holders of a majority in
principal amount of the outstanding debt securities of any series may, on behalf of holders of all those debt securities, waive any past default and consequences of such default.
The
indenture imposes limitations on suits brought by holders of debt securities against us. Except for actions for payment of overdue principal or interest, no holder of debt securities
of any series may institute any action against us under the indenture unless:
-
•
-
the holder has previously given to the trustee written notice of a continuing default;
15
Table of Contents
-
•
-
the holders of at least 25% in principal amount of the outstanding debt securities of the affected series have requested that the
trustee institute the action;
-
•
-
the requesting holders have offered the trustee indemnity or security for the costs, expenses and liabilities that may be incurred by
bringing the action;
-
•
-
the trustee has not instituted the action within 60 days of the request and offer of indemnity; and
-
•
-
the trustee has not received inconsistent direction by the holders of a majority in principal amount of the outstanding debt
securities of the affected series.
We
will be required to file annually with the trustee a certificate, signed by one of our officers, stating whether or not the officer knows of any default by us in the performance,
observance or fulfillment of any condition or covenant of the indenture.
Discharge, Defeasance and Covenant Defeasance
We can discharge or decrease our obligations under the indenture as stated below.
We
may discharge obligations to holders of any series of debt securities that have not already been delivered to the trustee for cancellation and that have either become due and payable
or are by their terms to become due and payable, or are scheduled for redemption, within one year. We may effect a discharge by irrevocably depositing with the trustee cash or government obligations
denominated in the currency of the debt securities, as trust funds, in an amount certified to be enough to pay when due, whether at maturity, upon redemption or otherwise, the principal of, and any
premium and interest on, the debt securities and any mandatory sinking fund payments.
Unless
otherwise provided in the applicable prospectus supplement, we may also discharge certain of our obligations to holders of any series of debt securities at any time, which we
refer to as defeasance. We may also be released from the obligations imposed by certain covenants of outstanding series of debt securities and provisions of the indenture, and we may omit to comply
with those covenants without creating an event of default under the indenture, which we refer to as covenant defeasance. We may effect defeasance and covenant defeasance only if, among other
things:
-
•
-
we irrevocably deposit with the trustee cash or government obligations denominated in the currency of the debt securities, as trust
funds, in an amount certified by a nationally recognized firm of independent certified accountants to be enough to pay at maturity, or upon redemption, the principal (including any mandatory sinking
fund payments) of, and any premium and interest on, all outstanding debt securities of the series; and
-
•
-
we deliver to the trustee an opinion of counsel to the effect that the holders of the series of debt securities will not recognize
income, gain or loss for U.S. federal income tax purposes as a result of the defeasance or covenant defeasance and that defeasance or covenant defeasance will not otherwise alter the holders' U.S.
federal income tax treatment of principal, and any premium and interest payments on, the series of debt securities.
In
the case of a defeasance by us, the opinion we deliver must be based on a ruling of the Internal Revenue Service issued, or a change in U.S. federal income tax law occurring, after
the date of the indenture.
Although
we may discharge or decrease our obligations under the indenture as described in the preceding paragraphs, we may not discharge certain enumerated obligations, including but not
limited to, our duty to register the transfer or exchange of any series of debt securities, to replace any temporary, mutilated, destroyed, lost or stolen series of debt securities or to maintain an
office or agency in respect of any series of debt securities.
16
Table of Contents
Modification of the Indenture
The indenture provides that we and the trustee may amend the indenture or enter into supplemental indentures without the consent of the
holders of debt securities to, among other things:
-
•
-
evidence the assumption by a successor entity of our obligations;
-
•
-
add to our covenants for the benefit of the holders of debt securities, or to surrender any rights or power conferred upon us;
-
•
-
add any additional events of default;
-
•
-
cure any ambiguity or correct any inconsistency or defect in the indenture provided that it does not adversely affect the interests of
the holders of any outstanding debt securities in any material respect;
-
•
-
add to, change or eliminate any of the provisions of the indenture in a manner that will become effective only when there is no
outstanding debt security which is entitled to the benefit of the provision as to which the modification would apply;
-
•
-
add guarantees to or secure any debt securities;
-
•
-
establish the forms or terms of debt securities of any series;
-
•
-
evidence and provide for the acceptance of appointment by a successor trustee and add to or change any of the provisions of the
indenture as is necessary for the administration of the trusts by more than one trustee;
-
•
-
add to or change any provision of the indenture as is necessary to permit or facilitate the issuance of debt securities in bearer
form;
-
•
-
change the location of (i) payment of principal, premium or interest; (ii) surrender of the debt securities for
registration, transfer or exchange and (iii) notices and demands to or upon us;
-
•
-
supplement any provision of the indenture to permit or facilitate the defeasance and discharge of any debt securities provided that it
does not adversely affect the interests of the holders of any outstanding debt securities in any material respect;
-
•
-
conform the terms of any debt securities to the description of such debt securities in the prospectus and prospectus supplement
offering the debt securities, as evidenced by an officer's certificate, provided that it does not adversely affect the interests of the holders of any outstanding debt securities in any material
respect;
-
•
-
eliminate any provision that was required at the time we entered into the indenture but, as a result of an amendment to the Trust
Indenture Act of 1939, as amended the Trust Indenture Act, is no longer required;
-
•
-
modify, eliminate or add to the provisions of the indenture to effect or evidence any change required by an amendment to the Trust
Indenture Act; and
-
•
-
make any other provisions with respect to matters or questions arising under the indenture as long as the new provisions do not
adversely affect the interests of the holders of any outstanding debt securities of any series created prior to the modification in any material respect.
Any
provision of the indenture shall automatically be deemed to have been modified, eliminated or added to the extent required to be made as a result of an amendment to the Trust
Indenture Act.
The
indenture also provides that we and the trustee may, with the written consent of the holders of not less than a majority in aggregate principal amount of debt securities of each
series of debt securities affected by such supplemental indenture then outstanding, add any provisions to, or change
17
Table of Contents
in
any manner, eliminate or modify in any way the provisions of, the indenture or any supplemental indenture or modify in any manner the rights of the holders of the debt securities. We and the
trustee may not, however, without the consent of the holder of each outstanding debt security affected thereby:
-
•
-
extend the final maturity of any debt security;
-
•
-
reduce the principal amount or premium, if any;
-
•
-
reduce the rate or extend the time of payment of interest;
-
•
-
change the method of calculating the rate of interest in a manner adverse to the holders of any outstanding debt securities;
-
•
-
reduce the amount of the principal of any debt security issued with an original issue discount that is payable upon acceleration;
-
•
-
change the currency in which the principal, and any premium or interest, is payable;
-
•
-
impair the right to institute suit for the enforcement of any payment on any debt security when due;
-
•
-
if applicable, adversely affect the right of a holder to convert or exchange a debt security; or
-
•
-
reduce the percentage of holders of debt securities of any series whose consent is required for any modification of the indenture or
for waivers of compliance with or defaults under the indenture with respect to debt securities of that series.
The
indenture provides that the holders of not less than a majority in aggregate principal amount of the then outstanding debt securities of any series, by written notice to the trustee,
may on behalf of the holders of the debt securities of that series waive any default and its consequences under the indenture except:
-
•
-
a default in the payment of the principal of or premium or interest on any such debt security; or
-
•
-
a default in respect of a covenant or provision of the indenture that cannot be modified or amended without the consent of the holder
of each outstanding debt security of each series affected.
Registered Global Securities and Book Entry System
The debt securities of a series may be issued in whole or in part in book-entry form and may be represented by one or more fully
registered global securities. We will deposit any registered global securities with a depositary or with a nominee for a depositary identified in the applicable prospectus supplement or with its
custodian and such global securities shall be registered in the name of such depositary or nominee. In such case, we will issue one or more registered global securities denominated in an amount equal
to the aggregate principal amount of all of the debt securities of the series to be issued and represented by such registered global security or securities. This means that we will not issue
certificates to each holder. See "Legal Ownership of Securities."
Unless
and until it is exchanged in whole or in part for debt securities in definitive registered form, a registered global security may not be transferred except as a
whole:
-
•
-
by the depositary for the registered global security to its nominee;
-
•
-
by a nominee of the depositary to the depositary or another nominee of the depositary; or
-
•
-
by the depositary or its nominee to a successor of the depositary or a nominee of the successor.
The
prospectus supplement relating to a series of debt securities will describe the specific terms of the depositary arrangement involving any portion of the series represented by a
registered global
18
Table of Contents
security.
We anticipate that the following provisions will apply to all depositary arrangements for debt securities:
-
•
-
ownership of beneficial interests in a registered global security will be limited to persons that have accounts with the depositary
for such registered global security, these persons being referred to as "participants," or persons that may hold interests through participants;
-
•
-
upon the issuance of a registered global security, the depositary for the registered global security will credit, on its book-entry
registration and transfer system, the participants' accounts with the respective principal amounts of the debt securities represented by the registered global security beneficially owned by the
participants;
-
•
-
any dealers, underwriters or agents participating in the distribution of the debt securities will designate the accounts to be
credited; and
-
•
-
ownership of beneficial interest in the registered global security will be shown on, and the transfer of the ownership interest will
be effected only through, records maintained by the depositary for the registered global security for interests of participants, and on the records of participants for interests of persons holding
through participants.
The
laws of some states may require that specified purchasers of securities take physical delivery of the securities in definitive form. These laws may limit the ability of those persons
to own, transfer or pledge beneficial interests in registered global securities.
So
long as the depositary for a registered global security, or its nominee, is the registered owner of the registered global security, the depositary or such nominee, as the case may be,
will be considered the sole owner or holder of the debt securities represented by the registered global security for all purposes under the indenture. Except as stated below, owners of beneficial
interests in a registered global security:
-
•
-
will not be entitled to have the debt securities represented by a registered global security registered in their names;
-
•
-
will not receive or be entitled to receive physical delivery of the debt securities in the definitive form; and
-
•
-
will not be considered the owners or holders of the debt securities under the indenture.
Accordingly,
each person owning a beneficial interest in a registered global security must rely on the procedures of the depositary for the registered global security and, if the person
is not a participant, on the procedures of a participant through which the person owns its interest, to exercise any rights of a holder under the indenture.
We
understand that under existing industry practices, if we request any action of holders or if an owner of a beneficial interest in a registered global security desires to give or take
any action that a holder is entitled to give or take under the indenture, the depositary for the registered global security would
authorize the participants holding the relevant beneficial interests to give or take the action, and the participants would authorize beneficial owners owning through the participants to give or take
the action or would otherwise act upon the instructions of beneficial owners holding through them.
We
will make or cause to be made payments of principal and premium, if any, and interest, if any, on debt securities represented by a registered global security registered in the name of
a depositary or its nominee to the depositary or its nominee, as the case may be, as the registered owners of the registered global security. Neither we nor the trustee, or any agent of ours or the
trustee will be responsible or liable for any aspect of the records relating to, or payments made on account of, beneficial ownership interests in the registered global security or for maintaining,
supervising or reviewing any records relating to the beneficial ownership interests.
19
Table of Contents
We expect that the depositary for any debt securities represented by a registered global security, upon receipt of any payments of principal and premium, if any,
and interest, if any, in respect of the registered global security, will immediately credit participants' accounts with payments in amounts proportionate to their respective beneficial interests in
the registered global security as shown on the records of the depositary. We also expect that standing customer instructions and customary practices will govern payments by participants to owners of
beneficial interests in the registered global security held through the participants, as is now the case with the securities held for the accounts of customers in bearer form or registered in "street
name." We also expect that any of these payments will be the responsibility of the participants.
If
the depositary for any debt securities represented by a registered global security is at any time unwilling or unable to continue as depositary, we will appoint an eligible successor
depositary. If we fail to appoint an eligible successor depositary within 90 days, or if an event of default has occurred and is continuing and the holders of a majority in aggregate principal
amount of the then outstanding debt securities of any series so request, we will issue the debt securities in definitive form in exchange for the registered global security. In addition, we may at any
time and in our sole discretion and subject to the depositary's procedures decide not to have any of the debt securities of a series represented by one or more registered global securities. In that
event, we will issue debt securities of the series in a definitive form in exchange for all of the registered global securities representing the debt securities.
The trustee will register any debt securities issued in definitive form in exchange for a registered global security in the name or names as the depositary, based upon instructions from its
participants, shall instruct the trustee.
We
may also issue bearer debt securities of a series in the form of one or more global securities, referred to as "bearer global securities." We will deposit these securities with a
depositary identified in the prospectus supplement relating to the series. The prospectus supplement relating to a series of debt securities represented by a bearer global security will describe the
applicable terms and procedures. These will include the specific terms of the depositary arrangement and any specific procedures for the issuance of debt securities in definitive form in exchange for
a bearer global security, in proportion to the series represented by a bearer global security.
Concerning the Trustee
The indenture provides that in the event that the trustee resigns or is removed with respect to less than all series of debt securities
outstanding under the indenture, there may be more than one trustee under the indenture. If there are different trustees for different series of debt securities under the indenture, each such trustee
will be a trustee of a trust under the indenture separate and apart from the trust administered by any other trustee under the indenture. Except as otherwise indicated in this prospectus or any
prospectus supplement, any action permitted to be taken by a trustee may be taken by such trustee only on the one or more series of debt securities for which it is the trustee under the indenture. Any
trustee under the indenture may resign or be removed from one or more series of debt securities.
The
indenture provides that, except during the continuance of an event of default, the trustee will perform only such duties as are specifically set forth in the indenture. During the
existence of an event of default, the trustee will exercise those rights and powers vested in it under the indenture and use the same degree of care and skill in its exercise as a prudent person would
exercise under the circumstances in the conduct of such person's own affairs.
The
trustee may engage in other transactions with us. If the trustee acquires any conflicting interest relating to any duties concerning the debt securities, however, the trustee must
eliminate the conflict or resign as trustee.
20
Table of Contents
No Individual Liability of Incorporators, Stockholders, Officers or Directors
The indenture provides that no past, present or future director, officer, stockholder or employee of ours, any of our affiliates, or
any successor corporation, in their capacity as such, shall have any individual liability for any of our obligations, covenants or agreements under the debt securities or the indenture.
Governing Law
The indenture is, and any debt securities will be, governed by, and construed in accordance with, the laws of the State of New York.
DESCRIPTION OF WARRANTS
General
The following description, together with the additional information we may include in any applicable prospectus supplement, summarizes
the material terms and provisions of the warrants that we may offer under this prospectus and the related warrant agreements and warrant certificates. While the terms summarized below will apply
generally to any warrants we may offer, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement. The form for each type of warrant will
be filed as an exhibit to the registration statement of which this prospectus is a part or will be incorporated by reference from a current report on Form 8-K that we file with the SEC.
We
may issue, together with other securities or separately, warrants to purchase preferred stock, common stock, depositary shares or debt securities. We will issue the warrants under
warrant agreements to be entered into between us and a bank or trust company, as warrant agent, all as shall be set forth in the applicable prospectus supplement. The warrant agent will act solely as
our agent in connection with the warrants of the series being offered and will not assume any obligation or relationship of agency or trust for or with any holders or beneficial owners of warrants.
Further
terms of the warrants will be set forth in the applicable prospectus supplement, including, where applicable, the following:
-
•
-
the title of such warrants;
-
•
-
the aggregate number of warrants;
-
•
-
the price or prices at which the warrants will be issued;
-
•
-
the designation, terms and number of shares of common stock, preferred stock, depositary shares or debt securities purchasable upon
exercise of the warrants;
-
•
-
the designation and terms of the securities with which the warrants are issued and the number of warrants issued with such securities;
-
•
-
the date on and after which the warrants and the related securities will be separately transferable, including any limitations on
ownership and transfer of the warrants;
-
•
-
in the case of warrants to purchase common stock, preferred stock, depositary shares or debt securities, the price at which each share
of common stock or preferred stock, each depositary share or each debt security purchasable upon exercise of the warrants may be purchased;
-
•
-
any provisions for adjustment of the number or amount of securities receivable upon exercise of the warrants;
-
•
-
the dates on which the right to exercise the warrants shall commence and expire;
21
Table of Contents
-
•
-
the minimum or maximum amount of warrants that may be exercised at any one time;
-
•
-
information with respect to book-entry procedures, if any;
-
•
-
a discussion of certain federal income tax consequences; and
-
•
-
any other terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants.
Before
exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including in the case of warrants to
purchase common stock, preferred stock or depositary shares, the right to receive dividends, if any, or payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any.
Exercise of Warrants
Each warrant will entitle the holder thereof to purchase for cash the securities at the exercise price as shall in each case be set
forth in, or be determinable as set forth in, the applicable prospectus supplement and warrant certificate. Warrants may be exercised at any time up to the close of business on the expiration date set
forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
Holders
of the warrants may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information, and paying the
required amount to the warrant agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth on the reverse side of the warrant certificate and in the
applicable prospectus supplement the information that the holder of the warrant will be required to deliver to the warrant agent.
Warrants
may be exercised as set forth in the applicable prospectus supplement relating to the warrants offered thereby. Upon receipt of payment of the exercise price and the warrant
certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will, as soon as
practicable, forward the purchased securities. If less than all of the warrants represented by the warrant certificate are exercised, a new warrant certificate will be issued for the remaining
warrants. If we so indicate in the applicable prospectus supplement, holders of the warrants may surrender securities as all or part of the exercise price for warrants.
Governing Law
Unless we provide otherwise in the applicable prospectus supplement, the warrants and warrant agreements, and any claim, controversy or
dispute arising under or related to the warrants or warrant agreements, will be governed by and construed in accordance with the laws of the State of New York.
Enforceability of Rights of Holders of Warrants
Each warrant agent will act solely as our agent under the applicable warrant agreement and will not assume any obligation or
relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as a warrant agent for more than one issue of warrants. A warrant agent will have no duty or
responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any
demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise, and receive
the securities purchasable upon exercise of, that holder's warrants.
22
Table of Contents
DESCRIPTION OF UNITS
The following description, together with the additional information we may include in any applicable prospectus supplement, summarizes
the material terms and provisions of the units that we may offer under this prospectus. While the terms we have summarized below will apply generally to any units that we may offer under this
prospectus, we will describe the particular terms of any series of units in more detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ
from the terms described below. However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer a security that is not registered and described in
this prospectus at the time of its effectiveness.
We
will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from a current report on Form 8-K that we file with the
SEC, the form of unit agreement that describes the terms of the series of units we are offering, and any supplemental agreements, before the issuance of the related series of units. The following
summaries of material terms and provisions of the units are subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement and any supplemental agreements
applicable to a particular series of units. We urge you to read the applicable prospectus supplement related to the particular series of units that we sell under this prospectus, as well as the
complete unit agreement and any supplemental agreements that contain the terms of the units.
General
We may issue units comprised of one or more debt securities, shares of common stock, shares of preferred stock and warrants in any
combination. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a
holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any
time before a specified date.
We
will describe in the applicable prospectus supplement the terms of the series of units, including:
-
•
-
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances
those securities may be held or transferred separately;
-
•
-
any provisions of the governing unit agreement that differ from those described below; and
-
•
-
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units.
The
provisions described in this section, as well as those described under "Description of Capital Stock," "Description of Depositary Shares," "Description of Debt Securities" and
"Description of Warrants" will apply to each unit and to any common stock, preferred stock, debt security or warrant included in each unit, respectively.
Governing Law
Unless we provide otherwise in the applicable prospectus supplement, the unit agreements, and any claim, controversy or dispute arising
under or related to the unit agreements, will be governed by and construed in accordance with the laws of the State of New York.
Issuance in Series
We may issue units in such amounts and in numerous distinct series as we determine.
23
Table of Contents
Enforceability of Rights by Holders of Units
Each unit agent will act solely as our agent under the applicable unit agreement and will not assume any obligation or relationship of
agency or trust with any holder of any unit. A single bank or trust company may act as unit agent for more than one series of units. A unit agent will have no duty or responsibility in case of any
default by us under the applicable unit agreement or unit, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a unit
may, without the consent of the related unit
agent or the holder of any other unit, enforce by appropriate legal action its rights as holder under any security included in the unit.
Title
We, the unit agents and any of their agents may treat the registered holder of any unit certificate as an absolute owner of the units
evidenced by that certificate for any purpose and as the person entitled to exercise the rights attaching to the units so requested, despite any notice to the contrary. See "Legal Ownership of
Securities."
LEGAL OWNERSHIP OF SECURITIES
We can issue securities in registered form or in the form of one or more global securities. We describe global securities in greater
detail below. We refer to those persons who have securities registered in their own names on the books that we or any applicable trustee, depositary or warrant agent maintain for this purpose as the
"holders" of those securities. These persons are the legal holders of the securities. We refer to those persons who, indirectly through others, own beneficial interests in securities that are not
registered in their own names, as "indirect holders" of those securities. As we discuss below, indirect holders are not legal holders, and investors in securities issued in book-entry form or in
street name will be indirect holders.
Book-Entry Holders
We may issue securities in book-entry form only, as we will specify in the applicable prospectus supplement. This means securities may
be represented by one or more global securities registered in the name of a financial institution that holds them as depositary on behalf of other financial institutions that participate in the
depositary's book-entry system. These participating institutions, which are referred to as participants, in turn, hold beneficial interests in the securities on behalf of themselves or their
customers.
Only
the person in whose name a security is registered is recognized as the holder of that security. Securities issued in global form will be registered in the name of the depositary or
its participants. Consequently, for securities issued in global form, we will recognize only the depositary as the holder
of the securities, and we will make all payments on the securities to the depositary. The depositary passes along the payments it receives to its participants, which in turn pass the payments along to
their customers who are the beneficial owners. The depositary and its participants do so under agreements they have made with one another or with their customers; they are not obligated to do so under
the terms of the securities.
As
a result, investors in a book-entry security will not own securities directly. Instead, they will own beneficial interests in a global security, through a bank, broker or other
financial institution that participates in the depositary's book-entry system or holds an interest through a participant. As long as the securities are issued in global form, investors will be
indirect holders, and not holders, of the securities.
24
Table of Contents
Street Name Holders
We may terminate a global security or issue securities in non-global form. In these cases, investors may choose to hold their
securities in their own names or in "street name." Securities held by an investor in street name would be registered in the name of a bank, broker or other financial institution that the investor
chooses, and the investor would hold only a beneficial interest in those securities through an account he or she maintains at that institution.
For
securities held in street name, we will recognize only the intermediary banks, brokers and other financial institutions in whose names the securities are registered as the holders of
those securities, and we will make all payments on those securities to them. These institutions pass along the payments they receive to their customers who are the beneficial owners, but only because
they agree to do so in their customer agreements or because they are legally required to do so. Investors who hold securities in street name will be indirect holders, not holders, of those securities.
Legal Holders
Our obligations, as well as the obligations of any applicable trustee and of any third parties employed by us or a trustee, run only to
the legal holders of the securities. We do not have obligations to investors who hold beneficial interests in global securities, in street name or by any other indirect means. This will be the case
whether an investor chooses to be an indirect holder of a security or has no choice because we are issuing the securities only in global form.
For
example, once we make a payment or give a notice to the holder, we have no further responsibility for the payment or notice even if that holder is required, under agreements with
depositary participants or customers or by law, to pass it along to the indirect holders but does not do so. Similarly, we may want to obtain the approval of the holders to amend an indenture, to
relieve us of the consequences of a default or of our obligation to comply with a particular provision of the indenture or for other purposes. In such an event, we would seek approval only from the
holders, and not the indirect holders, of the securities. Whether and how the holders contact the indirect holders is up to the holders.
Special Considerations For Indirect Holders
If you hold securities through a bank, broker or other financial institution, either in book-entry form or in street name, you should
check with your own institution to find out:
-
•
-
how it handles securities payments and notices;
-
•
-
whether it imposes fees or charges;
-
•
-
how it would handle a request for the holders' consent, if ever required;
-
•
-
whether and how you can instruct it to send you securities registered in your own name so you can be a holder, if that is permitted in
the future;
-
•
-
how it would exercise rights under the securities if there were a default or other event triggering the need for holders to act to
protect their interests; and
-
•
-
if the securities are in book-entry form, how the depositary's rules and procedures will affect these matters.
Global Securities
A global security is a security that represents one or any other number of individual securities held by a depositary. Generally, all
securities represented by the same global securities will have the same terms.
25
Table of Contents
Each
security issued in book-entry form will be represented by a global security that we deposit with and register in the name of a financial institution or its nominee that we select.
The financial
institution that we select for this purpose is called the depositary. Unless we specify otherwise in the applicable prospectus supplement, the Depository Trust Company will be the depositary for all
securities issued in book-entry form.
A
global security may not be transferred to or registered in the name of anyone other than the depositary, its nominee or a successor depositary, unless special termination situations
arise. We describe those situations below under "Special Situations When a Global Security Will Be Terminated." As a result of these arrangements, the depositary, or its nominee, will be the sole
registered owner and holder of all securities represented by a global security, and investors will be permitted to own only beneficial interests in a global security. Beneficial interests must be held
by means of an account with a broker, bank or other financial institution that in turn has an account with the depositary or with another institution that does. Thus, an investor whose security is
represented by a global security will not be a holder of the security, but only an indirect holder of a beneficial interest in the global security.
If
the prospectus supplement for a particular security indicates that the security will be issued in global form only, then the security will be represented by a global security at all
times unless and until the global security is terminated. If termination occurs, we may issue the securities through another book-entry clearing system or decide that the securities may no longer be
held through any book-entry clearing system.
Special Considerations For Global Securities
As an indirect holder, an investor's rights relating to a global security will be governed by the account rules of the investor's
financial institution and of the depositary, as well as general laws relating to securities transfers. We do not recognize an indirect holder as a holder of securities and instead deal only with the
depositary that holds the global security.
If
securities are issued only in the form of a global security, an investor should be aware of the following:
-
•
-
An investor cannot cause the securities to be registered in his or her name, and cannot obtain non-global certificates for his or her
interest in the securities, except in the special situations we describe below;
-
•
-
An investor will be an indirect holder and must look to his or her own bank or broker for payments on the securities and protection of
his or her legal rights relating to the securities, as we describe above;
-
•
-
An investor may not be able to sell interests in the securities to some insurance companies and to other institutions that are
required by law to own their securities in non-book-entry form;
-
•
-
An investor may not be able to pledge his or her interest in a global security in circumstances where certificates representing the
securities must be delivered to the lender or other beneficiary of the pledge in order for the pledge to be effective;
-
•
-
The depositary's policies, which may change from time to time, will govern payments, transfers, exchanges and other matters relating
to an investor's interest in a global security;
-
•
-
We and any applicable trustee have no responsibility for any aspect of the depositary's actions or for its records of ownership
interests in a global security, nor do we or the trustee supervise the depositary in any way;
26
Table of Contents
-
•
-
The depositary may, and we understand that the Depository Trust Company will, require that those who purchase and sell interests in a
global security within its book-entry system use immediately available funds, and your broker or bank may require you to do so as well; and
-
•
-
Financial institutions that participate in the depositary's book-entry system, and through which an investor holds its interest in a
global security, may also have their own policies affecting payments, notices and other matters relating to the securities.
There
may be more than one financial intermediary in the chain of ownership for an investor. We do not monitor and are not responsible for the actions of any of those intermediaries.
Special Situations When A Global Security Will Be Terminated
In a few special situations described below, the global security will terminate and interests in it will be exchanged for physical
certificates representing those interests. After that exchange, the choice of whether to hold securities directly or in street name will be up to the investor. Investors must consult their own banks
or brokers to find out how to have their interests in securities transferred to their own name, so that they will be direct holders. We have described the rights of holders and street name investors
above.
The
global security will terminate when the following special situations occur:
-
•
-
if the depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for that global security
and we do not appoint another institution to act as depositary within 90 days;
-
•
-
if we notify any applicable trustee that we wish to terminate that global security; or
-
•
-
if an event of default has occurred with regard to securities represented by that global security and has not been cured or waived.
The
prospectus supplement may also list additional situations for terminating a global security that would apply only to the particular series of securities covered by the prospectus
supplement. When a global security terminates, the depositary, and not we or any applicable trustee, is responsible for deciding the names of the institutions that will be the initial direct holders.
PLAN OF DISTRIBUTION
We may sell the securities being offered by this prospectus separately or together through any of the following
methods:
-
•
-
directly to purchasers;
-
•
-
through agents;
-
•
-
to or through one or more underwriters or dealers;
-
•
-
through a block trade in which the broker or dealer engaged to handle the block trade will attempt to sell the securities as agent,
but may position and resell a portion of the block as principal to facilitate the transaction; and
-
•
-
through a combination of any of these methods of sale.
We
may effect the distribution of the securities from time to time in one or more transactions:
-
•
-
at a fixed price or prices, which may be changed from time to time;
-
•
-
at market prices prevailing at the times of sale;
-
•
-
at prices related to such prevailing market prices; or
27
Table of Contents
We
may also distribute securities offered by this prospectus from time to time in one or more "at the market" offerings within the meaning of Rule 415(a)(4) of the Securities Act,
to or through a market maker, or into an existing trading market, on an exchange or otherwise. With our authorization, that market maker may also sell securities in privately negotiated transactions.
Each
time we offer a type or series of securities, we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms of the securities,
including, to the extent applicable:
-
•
-
the name or names of the underwriters, if any;
-
•
-
the purchase price of the securities or other consideration therefor, and the proceeds, if any, we will receive from the sale;
-
•
-
any over-allotment options under which underwriters may purchase additional securities from us;
-
•
-
any agency fees or underwriting discounts and other items constituting agents' or underwriters' compensation;
-
•
-
any public offering price;
-
•
-
any discounts or concessions allowed or reallowed or paid to dealers; and
-
•
-
any securities exchange or market on which the securities may be listed.
Only
underwriters named in the prospectus supplement will be underwriters of the securities offered by the prospectus supplement.
Agents.
We may solicit offers to purchase the securities offered by this prospectus through agents we designate from time to
time. We will name any
agent involved in the offer or sale of the securities and set forth any commissions payable by us to an agent in the applicable prospectus supplement. Unless otherwise indicated in the prospectus
supplement, any agent will be acting on a best efforts basis for the period of his or her appointment. Any agent may be deemed to be an "underwriter" of the securities as that term is defined in the
Securities Act of 1933, or the Securities Act.
Underwriters.
If we use an underwriter or underwriters in the sale of securities, we will execute an underwriting agreement with
the underwriter or
underwriters at the time we reach an agreement for sale. The underwriter or underwriters will acquire the securities for their own account and may resell the securities from time to time in one or
more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of an underwriter or underwriters to purchase the securities will be subject to
the conditions set forth in the applicable underwriting agreement. We will set forth in the prospectus supplement the names of the specific managing underwriter or underwriters, as well as any other
underwriters, and the terms of the transactions, including compensation of the underwriters and dealers. This compensation may be in the form of discounts, concessions or commissions. We may use
underwriters with whom we have a material relationship. Underwriters and others participating in any offering of the securities may engage in transactions that stabilize, maintain or otherwise affect
the price of the securities. We will describe any such relationship and any of these activities in the prospectus supplement.
Dealers.
If a dealer is used in the sale of the securities, an underwriter or we will sell securities to the dealer, as
principal. The dealer may
then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. The prospectus supplement will set forth the name of the dealer and the terms of the
transactions.
28
Table of Contents
Direct Sales.
We may directly solicit offers to purchase the securities, and we may sell directly to institutional investors or
others. These persons
may be deemed to be underwriters within the meaning of the Securities Act with respect to any resale of the securities. The prospectus supplement will describe the terms of any direct sales, including
the terms of any bidding or auction process.
Indemnification.
Agreements we enter into with agents, underwriters and dealers may entitle them to indemnification by us
against specified
liabilities, including liabilities under the Securities Act, or to contribution by us to payments they may be required to make in respect of these liabilities. The prospectus supplement will describe
the terms and conditions of indemnification or contribution.
Delayed Delivery Contracts.
We may authorize underwriters, dealers and agents to solicit offers by certain institutional
investors to purchase
offered securities under contracts providing for payment and delivery on a future date specified in the prospectus supplement. The prospectus supplement will also describe the public offering price
for the securities and the commission payable for solicitation of these delayed delivery contracts. Delayed delivery contracts will contain definite fixed price and quantity terms. The obligations of
a purchaser under these delayed delivery contracts will be subject to only two conditions:
-
•
-
that the institution's purchase of the securities at the time of delivery of the securities is not prohibited under the law of any
jurisdiction to which the institution is subject; and
-
•
-
that we shall have sold to the underwriters the total principal amount of the offered securities, less the principal amount covered by
the delayed delivery contracts.
Stabilization Activities.
To the extent permitted by and in accordance with Regulation M under the Securities Exchange Act
of 1934, as
amended, or the Exchange Act, in connection with an offering an underwriter may engage in over-allotments, stabilizing transactions, short covering transactions and penalty bids. Over-allotments
involve sales in excess of the offering size, which creates a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a
specified maximum. Short covering transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters
to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a covering transaction to cover short positions. Those activities may cause the price
of the securities to be higher than it would be otherwise. If commenced, the underwriters may discontinue any of these activities at any time.
Passive Market Making.
To the extent permitted by and in accordance with Regulation M under the Exchange Act, any
underwriters who are
qualified market makers on the Nasdaq Global Market may engage in passive market making transactions in the securities on the Nasdaq Global Market during the business day prior to the pricing of an
offering, before the commencement of offers or sales of the securities. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers.
In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker's
bid, however, the passive market maker's bid must then be lowered when certain purchase limits are exceeded.
Trading Markets and Listing.
Unless otherwise specified in the applicable prospectus supplement, each class or series of
securities will be a new
issue with no established trading market, other than our common stock, which is listed on the Nasdaq Global Market. We may elect to list any other class or series of securities on any exchange, but we
are not obligated to do so. It is possible that one or more underwriters may make a market in a class or series of securities, but the underwriters will not be obligated to do so and may discontinue
any market making at any time.
29
Table of Contents
We
cannot give any assurance as to the liquidity of the trading market for any of the securities we may offer under this prospectus.
In
compliance with guidelines of the Financial Industry Regulatory Authority, or FINRA, the maximum consideration or discount to be received by any FINRA member or independent broker
dealer may not exceed 8% of the aggregate amount of the securities offered pursuant to this prospectus and any applicable prospectus supplement.
No securities may be sold under this prospectus without delivery of an applicable prospectus supplement describing the method and terms of the
offering.
30
Table of Contents
LEGAL MATTERS
Gross Cutler Seiler Dupont LLC, Boulder, Colorado, will provide us with an opinion as to certain legal matters in connection
with the issuance and sale of the securities. Hogan Lovells US LLP, Denver, Colorado, will provide us with an opinion as to certain legal matters in connection with the issuance and sale of the
debt securities, the warrants and the units.
EXPERTS
The financial statements of Array BioPharma Inc. as of June 30, 2016 and 2015, and for each of the years in the
three-year period ended June 30, 2016, and management's assessment of the effectiveness of internal control over financial reporting as of June 30, 2016, have been incorporated by
reference herein and in the registration statement in reliance upon the reports of KPMG LLP, independent registered public accounting firm, incorporated by reference herein, and upon the
authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement under the Securities Act that registers the distribution of the securities offered
under this prospectus. The registration statement, including the attached exhibits and schedules and the information incorporated by reference, contains important information about our company and the
securities. The rules and regulations of the SEC allow us to omit from this prospectus certain information included in the registration statement. In addition, we file annual, quarterly and special
reports, proxy statements and other information with the SEC. You may read and copy this information and the registration statement at the SEC's Public Reference Room located at 100 F Street,
N.E., Washington D.C. 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the public reference room.
In
addition, any information we file with the SEC, including the registration statement and the documents incorporated by reference into this prospectus, and the exhibits thereto, is
also available on the SEC's website at http://www.sec.gov. We also maintain a web site at http://www.arraybiopharma.com, which provides additional information about our company and through which you
can also access our SEC filings. The information set forth on our web site is not part of this prospectus.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to incorporate by reference the information that we file with the SEC, which means that we can disclose important
information to you by referring you to those documents. The information incorporated by reference is considered to be part of this prospectus. These documents may include periodic reports, such as
Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as Proxy Statements. Any documents that we subsequently file with the SEC will
automatically update and replace the information previously filed with the SEC. Thus, for example, in the case of a conflict or inconsistency between information set forth in this prospectus and
information incorporated by reference into this prospectus, you should rely on the information contained in the document that was filed later.
This
prospectus incorporates by reference the documents listed below that we have previously filed with the SEC (under File No. 001-16633) and any additional documents that we may
file with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act between the date of this prospectus and the termination of the offering of the securities (other than any
documents or information deemed to have been furnished and not filed in accordance with SEC rules). These documents contain important information about us.
31
Table of Contents
-
•
-
Our Annual Report on Form 10-K for the fiscal year ended June 30, 2016 filed with the SEC on August 19, 2016;
-
•
-
Our Current Reports on Form 8-K, filed with the SEC on June 30, 2016, July 28, 2016, August 3, 2016 and
August 9, 2016.
-
•
-
The description of our common stock contained in our registration statement on Form 8-A filed with the SEC on
November 16, 2000, including any amendments or reports filed for the purpose of updating such description.
You
can obtain a copy of any or all of these documents, including any exhibits thereto, at no cost, by visiting the Investor Relations section of our web site at
http://www.arraybiopharma.com or by requesting them in writing or by telephone at the following address:
Array
BioPharma Inc.
3200 Walnut Street
Boulder, Colorado 80301
(303) 381-6600
Attention: Investor Relations
See
also the section entitled "Where You Can Find More Information" above.
Statements
contained in this prospectus and the documents incorporated by reference herein referring to the contents of any contract or other document are not necessarily complete. Where
such contract or other document is listed as an exhibit to the Registration Statement on Form S-3, of which this prospectus forms a part, or any document incorporated by reference
therein, each such statement is qualified by the provisions in such exhibit, to which reference is hereby made.
Information
contained on our website does not constitute a part of this prospectus.
32
Table of Contents
18,400,000 shares

Common stock
PROSPECTUS SUPPLEMENT
|
|
|
|
|
|
|
J.P. Morgan
|
|
|
|
Cowen and Company
|
Stifel
|
|
|
|
Wells Fargo Securities
|
SunTrust Robinson Humphrey
|
September 27, 2016
Array Technologies (NASDAQ:ARRY)
Historical Stock Chart
From Mar 2024 to Apr 2024
Array Technologies (NASDAQ:ARRY)
Historical Stock Chart
From Apr 2023 to Apr 2024