Oxford BioMedica Hails U.S. Regulatory Boost for Leukemia Treatment
March 30 2017 - 5:21AM
Dow Jones News
By Philip Waller
LONDON--Gene and cell therapy company Oxford BioMedica Plc
(OXB.LN) Thursday reported a regulatory boost linked to a product
that it supplies to Swiss drug company Novartis.
Oxford highlighted that Novartis had secured a fast-track review
in the U.S. for a treatment for leukemia in children and young
adults.
The U.S. Food & Drug Administration, or FDA, has granted
priority review for Novartis's CTL019 investigational chimeric
antigen receptor T-cell therapy, in relapsed and refractory
pediatric and young adult patients with B-cell acute lymphoblastic
leukemia.
The designation is expected to shorten the anticipated review
time by the FDA.
Oxford BioMedica is the only maker of the lentiviral vector
expressing CTL019 for Novartis.
Oxford's Chief Executive John Dawson said: "We continue to work
closely with Novartis in delivering the lentiviral vector
expressing CTL019, a product described earlier this year by
Novartis as having 'blockbuster' potential."
Shares in Oxford BioMedica were up 0.16 pence, or 3.2%, at 5.2
pence.
Write to Philip Waller at philip.waller@wsj.com
(END) Dow Jones Newswires
March 30, 2017 05:06 ET (09:06 GMT)
Copyright (c) 2017 Dow Jones & Company, Inc.
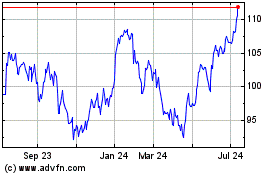
Novartis (NYSE:NVS)
Historical Stock Chart
From Mar 2024 to Apr 2024
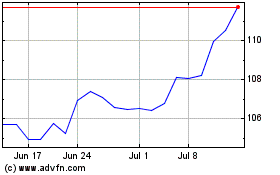
Novartis (NYSE:NVS)
Historical Stock Chart
From Apr 2023 to Apr 2024