FDA Panel Weighs Leukemia Gene-Therapy Treatment
July 12 2017 - 11:55AM
Dow Jones News
By Denise Roland
A group of cancer experts started weighing Wednesday whether to
recommend U.S. regulatory approval for a first-of-its kind gene
therapy targeting an aggressive form of leukemia that occurs in
children and young adults.
The treatment, called CTL019 and developed by Swiss drug giant
Novartis AG, works by modifying a patient's own immune cells to
make them more effective at hunting down and killing tumor cells.
It involves removing some of the patient's blood and genetically
modifying the T-cells, a type of immune cell, before
re-infusion.
A Food and Drug Administration advisory committee, which
comprises a group of cancer experts from across the U.S., will
determine whether the benefits of the therapy outweigh the risks.
The committee is expected to announce its decision late
Wednesday.
The FDA isn't required to follow the advice of such advisory
panels, but it generally does so. Analysts expect the FDA to issue
a final decision by the end of September.
The drug holds out the promise of a significant advance in the
treatment of children and young adults with a form of blood cancer
known as B-cell acute lymphoblastic leukemia. It is also the first
in a series of new treatments using genetically modified T-cells
facing FDA review.
For Novartis, a thumbs-up would open a potentially big, new
revenue stream. Jefferies analysts estimate the drug could bring in
$850 million in annual sales by 2021.
Novartis has said that in a clinical trial, 52 of 63
participants who received the treatment were cancer-free after
three months. The patients in the study had either failed to
respond to standard therapies, or relapsed.
Most children and young adults with this form of leukemia are
treated using existing methods such as chemotherapy, radiotherapy
and bone marrow transplants.
But the roughly one-in-seven patients who don't respond to
standard treatment, or suffer a relapse, have typically had only a
10% to 20% chance of survival, according to Rajesh Chopra, head of
cancer therapeutics at the Institute of Cancer Research in London.
The Novartis therapy increases that to around 80%. "That's big," he
said. Dr. Chopra isn't involved in the study.
CTL019 is the first of a hotly anticipated class of treatments
known as CAR-T therapies, all of which use genetically modified
T-cells, to be reviewed by the FDA. Hot on its heels is California
biotech Kite Pharma Inc., which filed its own CAR-T for non-Hodgkin
lymphoma with the FDA just days after Novartis.
Despite their promise, CAR-T therapies have also raised safety
concerns.
Seattle, Wash.-based Juno Therapeutics Inc. dropped its most
advanced CAR-T candidate earlier this year after several patient
deaths during a clinical trial.
A spokesman for Juno said the company had conducted an
exhaustive investigation into the causes of the deaths, which
appeared to be linked to a mix of the specific design of the
treatment, the characteristics of the patients and the process used
to administer the therapy. The company plans to release the
detailed results of its investigation at a scientific conference
later this year, he said.
He added that patients in an early-stage trial for its
now-leading product, JCAR017, experienced relatively low rates of
serious side effects.
A common side effect of CAR-T therapy is a condition known as
cytokine release syndrome, which causes severe flulike symptoms.
Novartis said most patients in its trial suffered some form of CRS,
but that none died from it.
The FDA, in briefing documents issued ahead of Wednesday's panel
meeting, also said there was a risk that CAR-T therapy could, in
the long term, make the modified T-cells cancerous.
That caution arises from an incident in the late 2000s when
several people developed a leukemia-like condition around 10 years
after receiving an older form of gene therapy for an
immunodeficiency disease. So far, there is no evidence that a
similar effect could occur with the Novartis therapy, which uses a
different form of virus to insert the new genetic material.
Xiaobin Victor Lu, gene therapy product reviewer at the FDA,
said during the hearing that the design of the Novartis therapy
lowered the chances of such a side effect, but that the risk
"cannot be entirely eliminated."
Write to Denise Roland at Denise.Roland@wsj.com
(END) Dow Jones Newswires
July 12, 2017 11:40 ET (15:40 GMT)
Copyright (c) 2017 Dow Jones & Company, Inc.
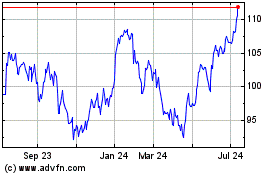
Novartis (NYSE:NVS)
Historical Stock Chart
From Mar 2024 to Apr 2024
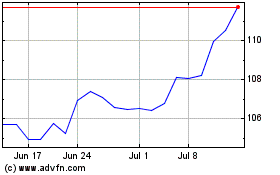
Novartis (NYSE:NVS)
Historical Stock Chart
From Apr 2023 to Apr 2024