Bayer's EYLEA Gets EU Approval for Retinal Vein Occlusion Treatment
February 26 2015 - 9:43AM
Dow Jones News
By Neetha Mahadevan
FRANKFURT--Germany's Bayer AG (BAYRY) said Thursday that its eye
treatment EYLEA received approval from the European Commission for
the treatment of retinal vein occlusion.
The drug has already been approved in many countries for
age-related macular degeneration, or wet AMD, and to treat impaired
vision due to macular edema secondary to central retinal vein
occlusion, or CRVO.
Bayer HealthCare and Regeneron Pharmaceuticals Inc. (REGN)
cooperate on the global development of EYLEA, one of Bayer's
blockbuster drugs. Regeneron maintains exclusive rights to EYLEA in
the U.S., while Bayer HealthCare has exclusive marketing license
rights elsewhere. The companies equally share in the profits from
sales of EYLEA, except in Japan, where Regeneron receives a
percentage of net sales.
Write to Neetha Mahadevan at neetha.mahadevan@wsj.com
Subscribe to WSJ: http://online.wsj.com?mod=djnwires
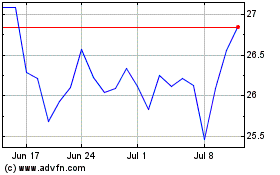
Bayer (TG:BAYN)
Historical Stock Chart
From Mar 2024 to Apr 2024
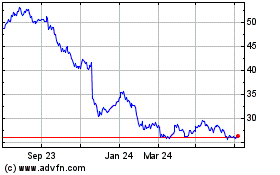
Bayer (TG:BAYN)
Historical Stock Chart
From Apr 2023 to Apr 2024