UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant
to Section 13 OR 15(d)
of the Securities Exchange Act of 1934
Date of report (date of earliest event reported): November 11, 2015
HEARTWARE INTERNATIONAL, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-34256 |
|
26-3636023 |
| (State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(I.R.S. Employer
Identification No.) |
500 Old Connecticut Path
Framingham, MA 01701
(Address of principal executive offices)
Registrant’s telephone number, including area code: 508.739.0950
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the
following provisions:
| x |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
On November 11, 2015, HeartWare International, Inc. (“HeartWare”)
presented at the Credit Suisse 24th Annual Healthcare Conference held in Scottsdale, Arizona. One of the topics covered in HeartWare’s presentation was HeartWare’s proposed acquisition
of Valtech Cardio, Ltd. (“Valtech”). A live webcast of HeartWare’s presentation was provided through the Company’s investor relations website and is available for replay at ir.heartware.com. Further,
HeartWare’s slide presentation used at the conference is attached hereto as Exhibit 99.1 and is hereby incorporated by reference.
As
previously announced, HeartWare intends to acquire Valtech as set forth in that certain Business Combination Agreement, dated as of September 1, 2015, by and among HeartWare, Valtech, HW Global, Inc., a Delaware corporation and a direct wholly
owned subsidiary of HeartWare (“Holdco”), HW Merger Sub, Inc., a Delaware corporation and a direct wholly owned subsidiary of Holdco (“US Merger Sub”), Valor Merger Sub Ltd., a private company incorporated under the
laws of Israel and a direct wholly owned subsidiary of Holdco (“ISR Merger Sub”) and Valor Shareholder Representative, LLC, a Delaware limited liability company, pursuant to which, subject to satisfaction or waiver of the conditions
therein, HeartWare and Valtech will effect a strategic combination of their respective businesses under Holdco wherein (a) US Merger Sub shall merge with and into HeartWare, with HeartWare surviving the merger as a wholly owned subsidiary of
Holdco (the “US Merger”), and (b) ISR Merger Sub shall merge with and into Valtech, with Valtech surviving the merger as a subsidiary of Holdco (the “ISR Merger,” together with the US Merger and the other
transactions contemplated by the Business Combination Agreement, the “Transactions”).
Additional information concerning the proposed
Transactions is included in the preliminary proxy statement/prospectus, which was filed by Holdco with the Securities and Exchange Commission on October 16, 2015.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
|
|
|
| Exhibit
No. |
|
Description |
|
|
| 99.1 |
|
November 11, 2015 HeartWare Slide Presentation for the Credit Suisse 24th Annual Healthcare Conference. |
Important Information
Additional Information about the Transactions and Where to Find It
In connection with the proposed Transactions, Holdco has filed a Registration Statement on Form S-4 that contains a preliminary proxy statement/prospectus,
which is not yet final and will be amended. Holdco intends to file a final prospectus and other relevant materials and HeartWare intends to file a definitive proxy statement and other relevant materials with the SEC in connection with the proposed
Transactions. Investors and security holders of HeartWare and Valtech are urged to read these materials when they become available because they will contain important information about HeartWare, Valtech and the Transactions. The proxy
statement/prospectus and other relevant materials (when they become available), and any other documents filed by Holdco or HeartWare with the SEC, may be obtained free of charge at the SEC website at www.sec.gov. In addition, investors and security
holders may obtain free copies of the documents filed with the SEC by Holdco or HeartWare by directing a written request to HeartWare’s investor relations department at HeartWare International, Inc., 500 Old Connecticut Path, Framingham, MA
01701, Attention: Investor Relations. Investors and security holders are urged to read the proxy statement/prospectus and the other relevant materials when they become available before making any voting or investment decision with respect to the
Transactions.
This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any
securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of
securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act.
Participants in the
Solicitation
HeartWare, Valtech and their respective directors, executive officers, certain members of management and certain employees may be deemed
to be participants in the solicitation of proxies from the stockholders of HeartWare and Valtech in connection with the proposed transaction. Information regarding the special interests of HeartWare’s directors and executive officers in the
transaction is included in the proxy statement/prospectus referred to above. Additional information regarding the directors and executive officers of HeartWare is also included in the HeartWare Annual Report on Form 10-K for the year ended
December 31, 2014, which was filed with the SEC on March 2, 2015. This document is available free of charge at the SEC website (www.sec.gov) and from Investor Relations at HeartWare at the address described above.
Forward-Looking Statements
This Current Report on Form
8-K contains forward-looking statements within the meaning of the Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements involve certain risks and
uncertainties that could cause actual results to differ materially from those indicated in such forward-looking statements, including, but not limited to, the ability of the parties to consummate the proposed Transactions; satisfaction of closing
conditions to the consummation of the proposed Transactions; and such other risks and uncertainties pertaining to HeartWare’s business as detailed in its filings with the SEC on Forms 10-K and 10-Q, which are available on the SEC’s website
at www.sec.gov. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date thereof. HeartWare assumes no obligation to update any forward-looking statement contained in this document.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned
hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
HeartWare International, Inc. |
|
|
|
|
| Date: November 12, 2015 |
|
|
|
By: |
|
/s/ Lawrence J. Knopf |
|
|
|
|
|
|
Name: Lawrence J. Knopf |
|
|
|
|
|
|
Title: Senior Vice President, General Counsel and Secretary |
INDEX TO EXHIBITS
|
|
|
| Exhibit
No. |
|
Description |
|
|
| 99.1 |
|
November 11, 2015 HeartWare Slide Presentation for the Credit Suisse 24th Annual Healthcare Conference. |
Exhibit 99.1


Credit Suisse 24th Annual Healthcare Conference November 11, 2015 · Scottsdale, AZ DOUG GODSHALL PRESIDENT AND CEO HEARTWARE, HVAD and the HEARTWARE logo are registered
trademarks of HeartWare.

Safe Harbor Statement Forward-Looking Statements This announcement contains forward-looking statements that are based on management’s beliefs, assumptions and expectations and on
information currently available to management. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation our
expectations with respect to the: commercialization of the HeartWare HVAD System and introduction of the MVAD System; timing, progress and outcomes of clinical trials; regulatory and quality compliance; research and development activities;
consummation of our proposed acquisition of Valtech and our ability to take advantage of acquired and pipeline technology. Management believes that these forward-looking statements are reasonable as and when made. However, you should not place undue
reliance on forward-looking statements because they speak only as of the date when made. HeartWare does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or
otherwise, except as may be required by federal securities laws and the rules and regulations of the Securities and Exchange Commission. HeartWare may not actually achieve the plans, projections or expectations disclosed in forward-looking
statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, including without limitation those
described in Part I, Item 1A. “Risk Factors” in HeartWare’s Annual Report on Form 10-K filed with the Securities and Exchange Commission. HeartWare may update risk factors from time to time in Part II, Item 1A. “Risk
Factors” in Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, or other filings with the Securities and Exchange Commission. HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.

Additional Shareholder Information Participants in the Solicitation HeartWare, Valtech and their respective directors, executive officers, certain members of management and certain
employees may be deemed to be participants in the solicitation of proxies in connection with the proposed acquisition of Valtech Cardio, Ltd. A description of the interests in HeartWare of its directors and executive officers is set forth in
HeartWare’s proxy statement for its 2015 Annual Meeting of Shareholders, which was filed with the Securities and Exchange Commission (the “SEC”) on April 30, 2015. Additional information regarding the persons who may, under the
rules of the SEC, be deemed participants in the solicitation of proxies in connection with the proposed transaction, and a description of their direct and indirect interests in the proposed transaction, which may differ from the interests of
HeartWare stockholders or Valtech shareholders generally, are set forth in a preliminary proxy statement/prospectus filed with the SEC on October 16, 2015. Additional Information and Where To Find It In connection with the proposed
transactions, HW Global, Inc. (“Holdco”), has filed a Registration Statement on Form S-4 that contains a preliminary proxy statement/prospectus, which is not yet final and will be amended. Holdco intends to file a final prospectus and
other relevant materials and HeartWare intends to file a definitive proxy statement and other relevant materials with the SEC in connection with the proposed transactions. Investors and security holders of HeartWare and Valtech are urged to read
these materials (when they become available) before making any voting or investment decision with respect to the transactions because they will contain important information about HeartWare, Valtech and the transactions. The proxy
statement/prospectus and other relevant materials, and any other documents filed by Holdco or HeartWare with the SEC, may be obtained free of charge at the SEC website at www.sec.gov. In addition, investors and security holders may obtain free
copies of these documents by directing a written request to HeartWare’s investor relations department at HeartWare International, Inc., 500 Old Connecticut Path, Framingham, MA 01701, Attention: Investor Relations. This communication shall not
constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful
prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended
(the “Securities Act”). HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.

Mission We create revolutionary technology for the treatment of heart failure to allow patients to get back to life. Note: Data as of November 2, 2015. 9,000+ ~125 Patients U.S.
Implanted Centers Globally ~180 47 Intl. Countries Centers 2009: CE Mark 2012: FDA Approval HVAD® System—A Proven Track Record HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.

Enabling Patients to Get Back to Life HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
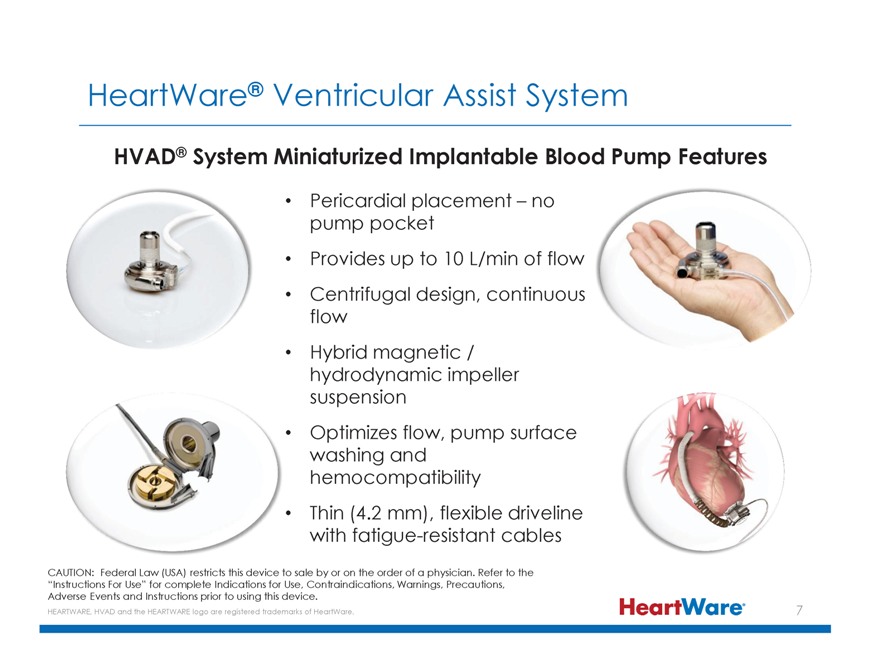
HeartWare® Ventricular Assist System HVAD® System Miniaturized Implantable Blood Pump Features Pericardial placement – no pump pocket Provides up to 10 L/min of flow
Centrifugal design, continuous flow Hybrid magnetic / hydrodynamic impeller suspension Optimizes flow, pump surface washing and hemocompatibility Thin (4.2 mm), flexible driveline with fatigue-resistant cables CAUTION: Federal Law (USA) restricts
this device to sale by or on the order of a physician. Refer to the “Instructions For Use” for complete Indications for Use, Contraindications, Warnings, Precautions, Adverse Events and Instructions prior to using this device. HEARTWARE,
HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
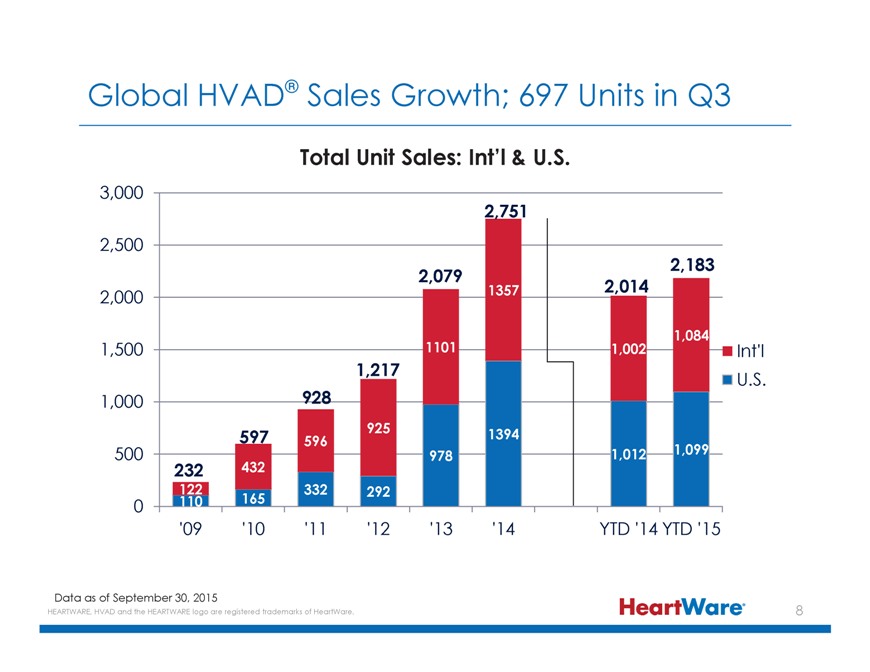
Global HVAD® Sales Growth; 697 Units in Q3 Total Unit Sales: Int’l & U.S. 3,000 2,751 2,500 2,183 2,079 1357 2,014 2,000 1,084 1,500 1101 1,002 Int’l 1,217 U.S.
1,000 928 597 925 1394 596 500 978 1,012 1,099 232 432 122 332 292 0 110 165 ‘09 ‘10 ‘11 ‘12 ‘13 ‘14 YTD ‘14 YTD ‘15 Data as of September 30, 2015 HEARTWARE, HVAD and the HEARTWARE logo are registered
trademarks of HeartWare.

Recent Milestones Announced agreement to acquire Valtech Cardio Commenced MVAD® System CE Mark international trial Completed enrollment in ENDURANCE2 DT trial Submitted MVAD IDE to FDA
Valtech’s Cardioband ® awarded CE Mark approval Continued ramping enrollment in HVAD® LATERAL; thoracotomy study ~60% enrolled Approaching 3 years of HVAD commercialization in U.S. Longest-supported HVAD patient reached 8 years on
support HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.

PIPELINE TECHNOLOGY HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
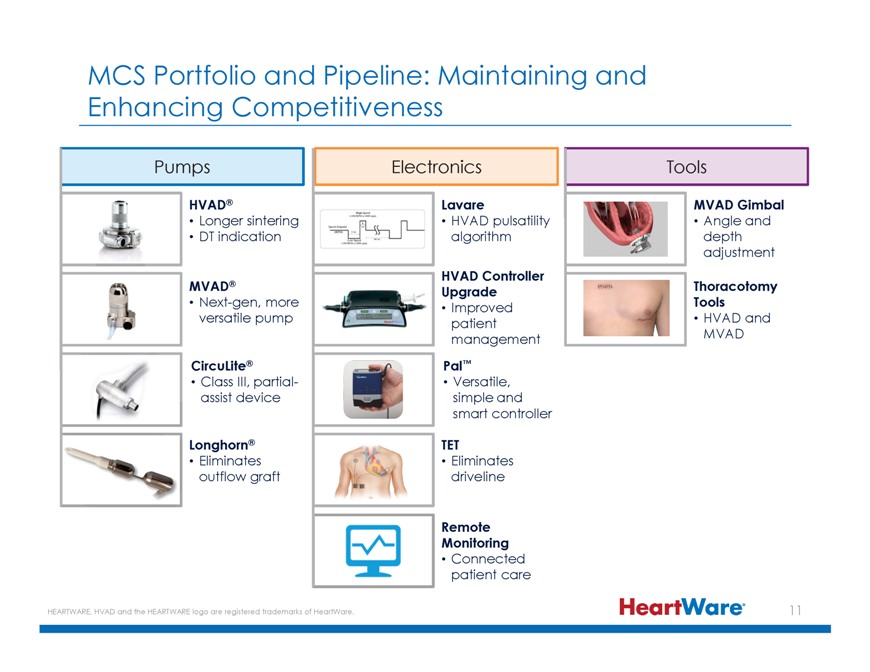
MCS Portfolio and Pipeline: Maintaining and Enhancing Competitiveness Pumps Electronics Tools HVAD® Lavare MVAD Gimbal Longer sintering HVAD pulsatility Angle and DT indication
algorithm depth adjustment HVAD Controller MVAD® Upgrade Thoracotomy Next-gen, more Improved Tools versatile pump patient • HVAD and management MVAD CircuLite® Pal™ Class III, partial- Versatile, assist device simple and smart
controller Longhorn® TET Eliminates Eliminates outflow graft driveline Remote Monitoring Connected patient care HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
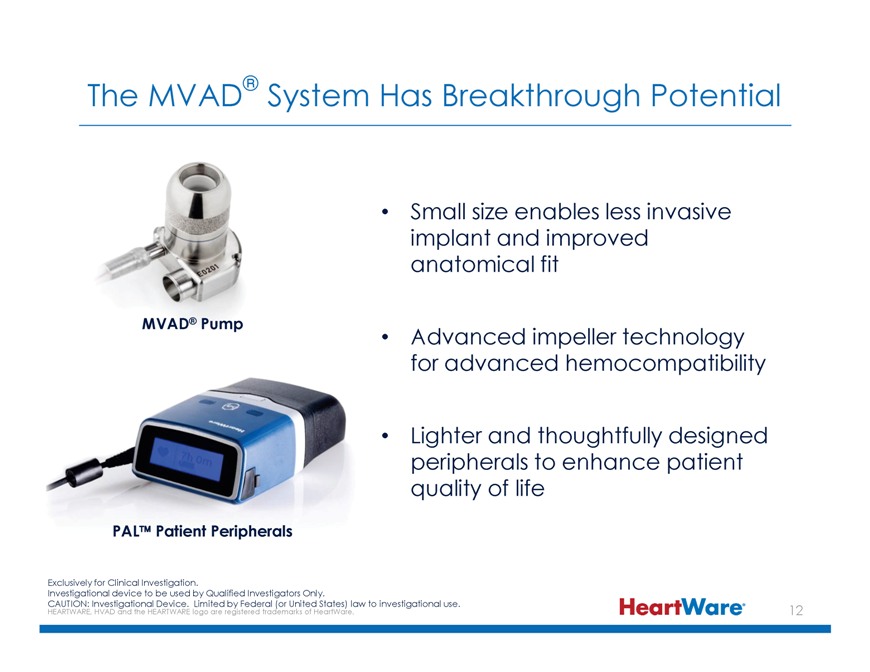
The MVAD® System Has Breakthrough Potential Small size enables less invasive implant and improved anatomical fit MVAD® Pump Advanced impeller technology for advanced
hemocompatibility Lighter and thoughtfully designed peripherals to enhance patient quality of life PAL™ Patient Peripherals Exclusively for Clinical Investigation. Investigational device to be used by Qualified Investigators Only. CAUTION:
Investigational Device. Limited by Federal (or United States) law to investigational use. HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
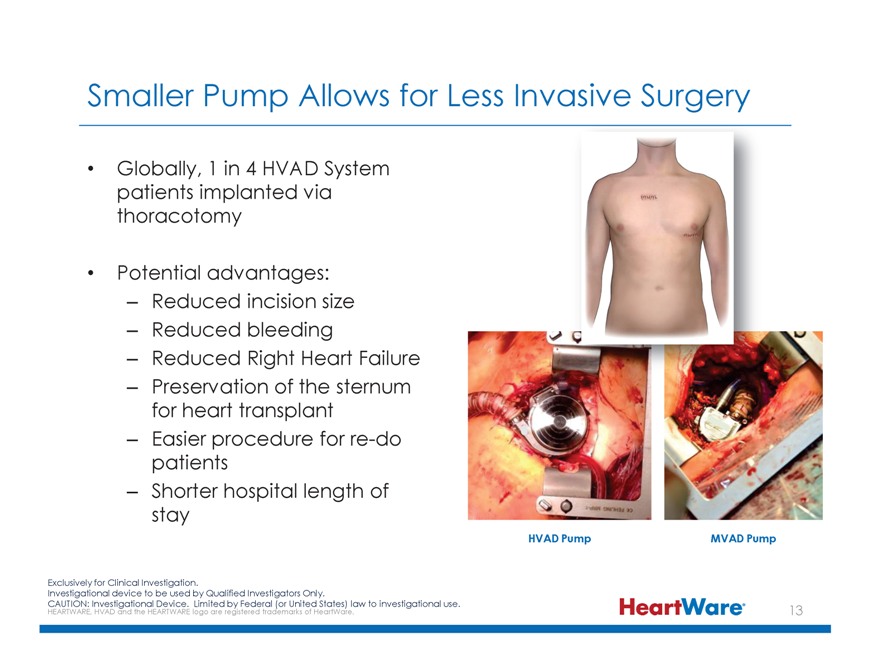
Smaller Pump Allows for Less Invasive Surgery Globally, 1 in 4 HVAD System patients implanted via thoracotomy Potential advantages: – Reduced incision size – Reduced bleeding
– Reduced Right Heart Failure – Preservation of the sternum for heart transplant – Easier procedure for re-do patients – Shorter hospital length of stay HVAD Pump MVAD Pump Exclusively for Clinical Investigation. Investigational
device to be used by Qualified Investigators Only. CAUTION: HEARTWARE, Investigational HVAD and the HEARTWARE Device. logo Limited are registered by Federal trademarks (or United of HeartWare States) law . to investigational use.
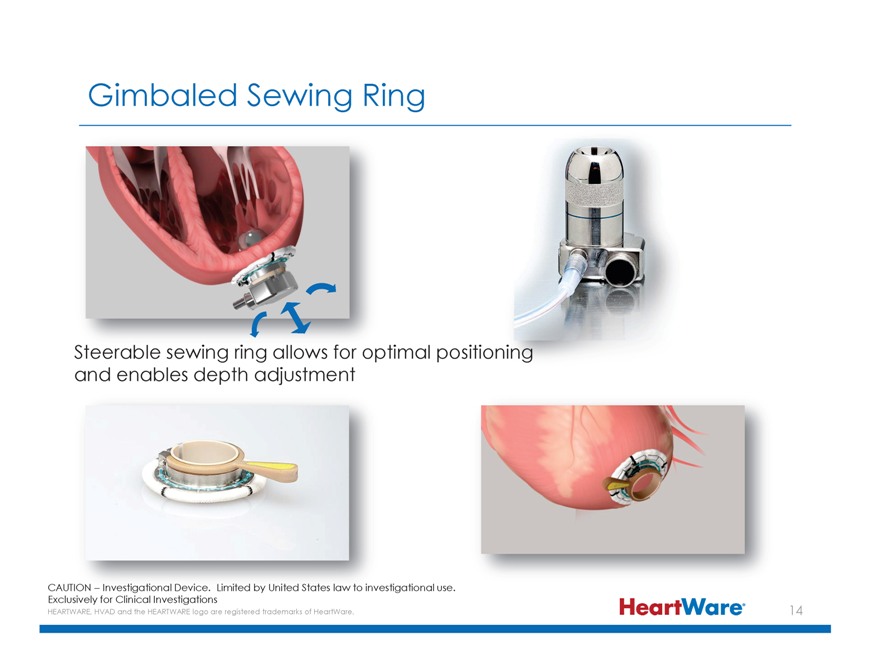
Gimbaled Sewing Ring Steerable sewing ring allows for optimal positioni and enables depth adjustment CAUTION – Investigational Device. Limited by United States law to investigational
use. Exclusively for Clinical Investigations HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
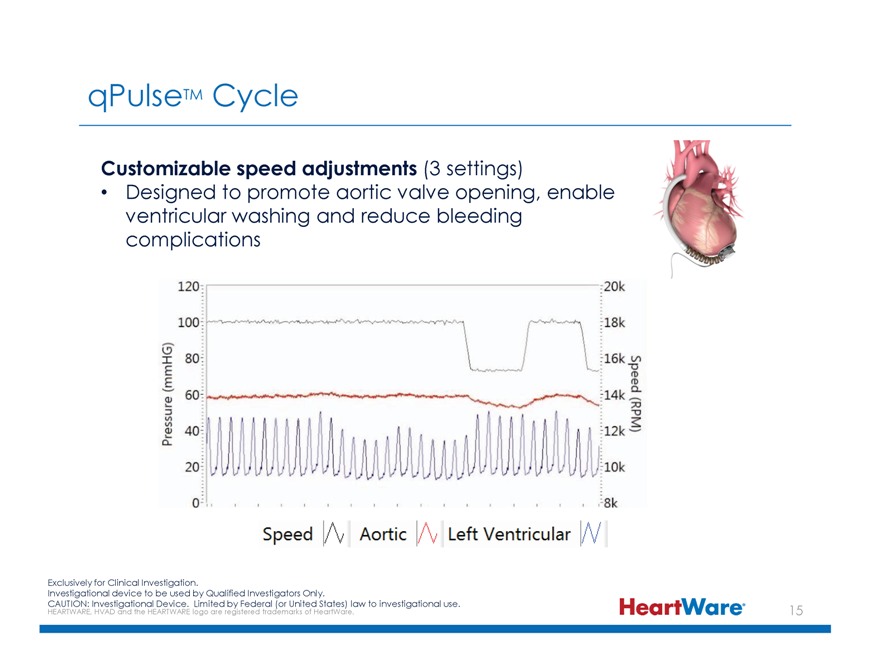
qPulseTM Cycle Customizable speed adjustments (3 settings) Designed to promote aortic valve opening, enable ventricular washing and reduce bleeding complications Exclusively for Clinical
Investigation. Investigational device to be used by Qualified Investigators Only. CAUTION: HEARTWARE, Investigational HVAD and the HEARTWARE Device. logo Limited are registered by Federal trademarks (or United of HeartWare. States) law to
investigational use.
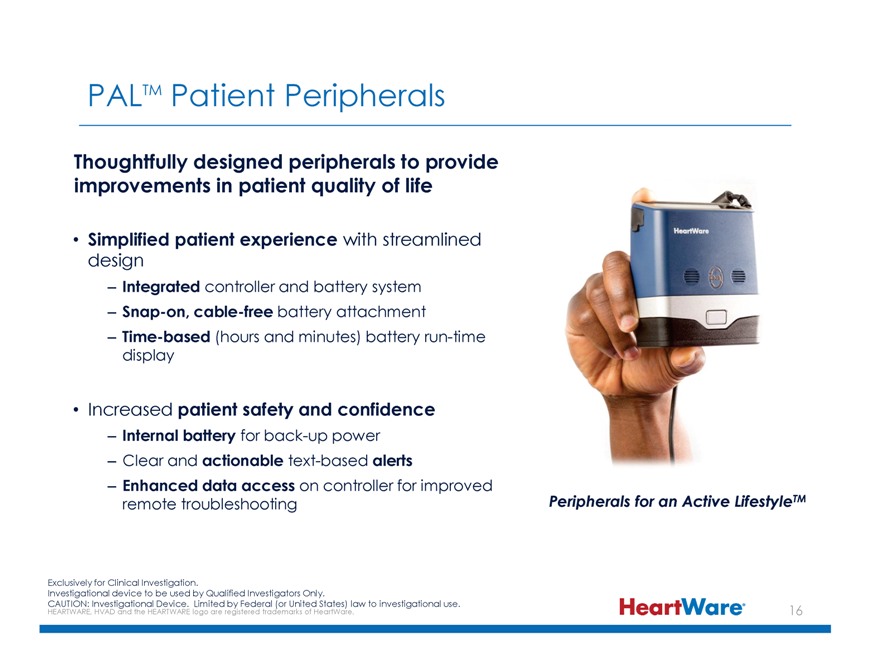
PALTM Patient Peripherals Thoughtfully designed peripherals to provide improvements in patient quality of life Simplified patient experience with streamlined design –Integrated
controller and battery system –Snap-on, cable-free battery attachment –Time-based (hours and minutes) battery run-time display Increased patient safety and confidence –Internal battery for back-up power – Clear and actionable
text-based alerts –Enhanced data access on controller for improved remote troubleshooting Peripherals for an Active LifestyleTM Exclusively for Clinical Investigation. Investigational device to be used by Qualified Investigators Only. CAUTION:
HEARTWARE, Investigational HVAD and the HEARTWARE Device. logo Limited are registered by Federal trademarks (or United of HeartWare. States) law to investigational use.
MVAD® Advantage™ CE Mark Trial Multi-center, prospective, non-randomized, single-arm trial 70 patients at 11 sites in Australia, Austria, France, Germany & the UK Primary
Endpoint: Survival at 6 months Implantation via thoracotomy or sternotomy First patient enrolled July 2015 Exclusively for Clinical Investigation. Investigational device to be used by Qualified Investigators Only. CAUTION: HEARTWARE, Investigational
HVAD and the HEARTWARE Device. logo Limited are registered by Federal trademarks (or United of HeartWare. States) law to investigational use.
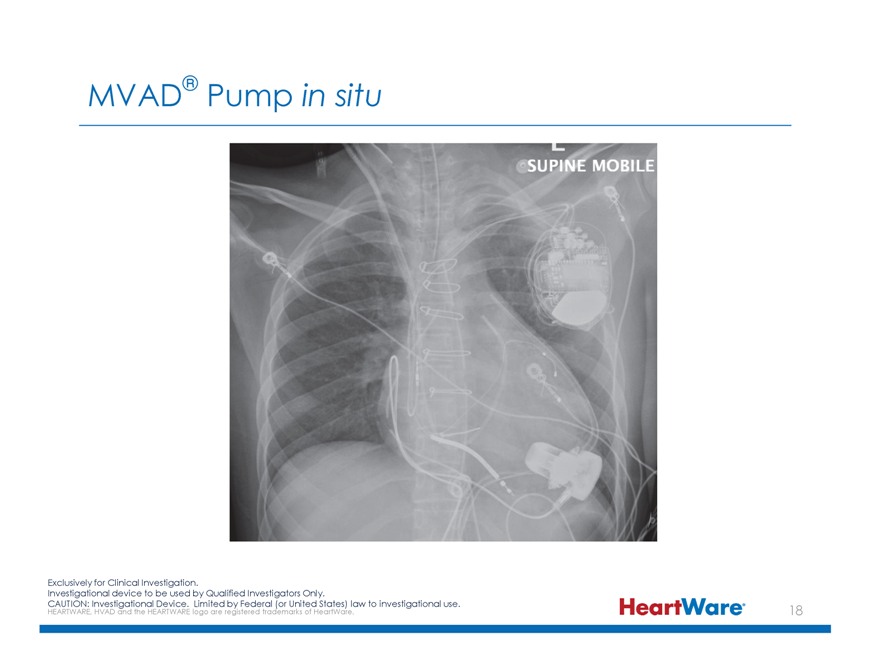
MVAD® Pump in situ Exclusively for Clinical Investigation. Investigational device to be used by Qualified Investigators Only. CAUTION: HEARTWARE, Investigational HVAD and the HEARTWARE
Device. logo Limited are registered by Federal trademarks (or United of HeartWare. States) law to investigational use.
MVAD® System Commentary Controller assembly fix complete, software patch progressing toward submission efficiently Investigation continues with no anticipated design modifications
Narrowing our focus to specific areas within our manufacturing process, which we may elect to further tighten up Working with investigators to review status and develop restart plan Finalizing decision tree for return to the clinic HEARTWARE, HVAD
and the HEARTWARE logo are registered trademarks of HeartWare.

BUSINESS COMBINATION HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
HeartWare + Valtech: Creating the Technology Leader in Heart Failure MVAD® System will lead a transformation of MCS portfolio and VAD market, picking up where HVAD® leaves off
Synergies with disease, customer, referral channel and delivery system will create compounding benefits over next decade Adjustable repair of MR and TR expected to emerge as first-line option, whether surgical or interventional Nearer-term
commercial opportunity in established surgical and interventional mitral repair market with >$450M in sales Sophisticated CardioValve™ design and delivery system to enable HeartWare leadership in mitral replacement Combined pipeline creates
unique opportunity for differentiated value creation HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare. Commercial Execution Excellence: Strong Commercial and Clinical Team Accustomed to Breaking Into Competitive Markets
Broad Global Presence HVAD® is the most-implanted LVAD internationally for the past 3 years Industry-leading sales and marketing serving top global heart centers 25% of sales team, including both North American and International Senior Directors
of Sales, has deep valve experience Highly skilled clinical specialists, many with advanced technical or clinical degrees Best-in-class distributor partners with backgrounds in interventional cardiology and structural heart HEARTWARE, HVAD and the
HEARTWARE logo are registered trademarks of HeartWare.
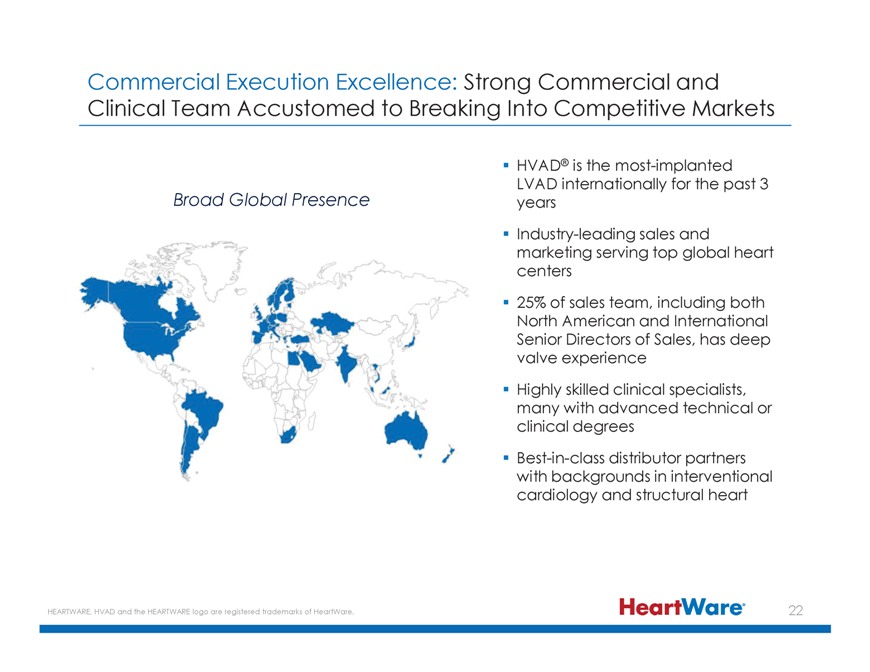
Commercial Execution Excellence: Strong Commercial and Clinical Team Accustomed to Breaking Into Competitive Markets HVAD® is the most-implanted LVAD internationally for the past 3
Broad Global Presence years Industry-leading sales and marketing serving top global heart centers 25% of sales team, including both North American and International Senior Directors of Sales, has deep valve experience Highly skilled clinical
specialists, many with advanced technical or clinical degrees Best-in-class distributor partners with backgrounds in interventional cardiology and structural heart
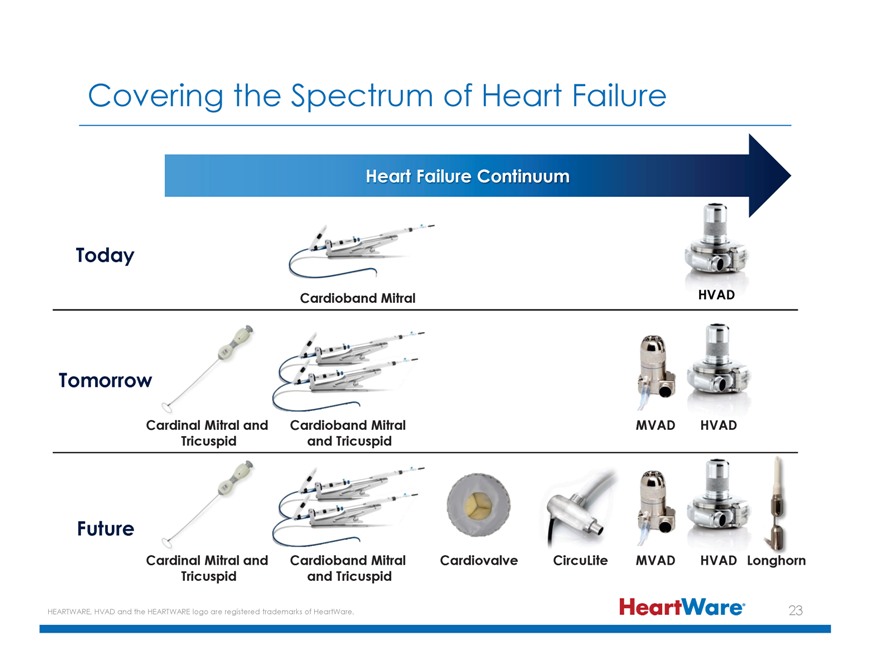
Covering the Spectrum of Heart Failure Heart Failure Continuum Today Cardioband Mitral Tomorrow Cardinal Mitral and Cardioband Mitral MVAD HVAD Tricuspid and Tricuspid Future Cardinal
Mitral and Cardioband Mitral Cardiovalve CircuLite MVAD HVAD Longhorn Tricuspid and Tricuspid HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare. 23
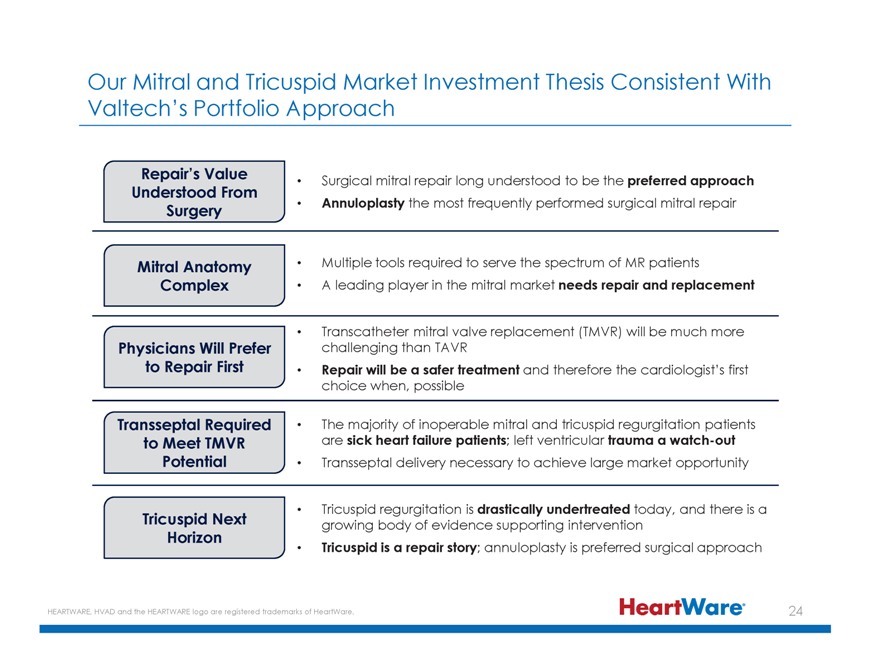
Our Mitral and Tricuspid Market Investment Thesis Consistent With Valtech’s Portfolio Approach Repair’s Value Surgical mitral repair long understood to be the preferred approach
Understood From Surgery Annuloplasty the most frequently performed surgical mitral repair Mitral Anatomy Multiple tools required to serve the spectrum of MR patients Complex A leading player in the mitral market needs repair and replacement
Transcatheter mitral valve replacement (TMVR) will be much more Physicians Will Prefer challenging than TAVR to Repair First Repair will be a safer treatment and therefore the cardiologist’s first choice when, possible Transseptal Required The
majority of inoperable mitral and tricuspid regurgitation patients to Meet TMVR are sick heart failure patients; left ventricular trauma a watch-out Potential Transseptal delivery necessary to achieve large market opportunity Tricuspid regurgitation
is drastically undertreated today, and there is a Tricuspid Next growing body of evidence supporting intervention Horizon Tricuspid is a repair story; annuloplasty is preferred surgical approach HEARTWARE, HVAD and the HEARTWARE logo are registered
trademarks of HeartWare. 2
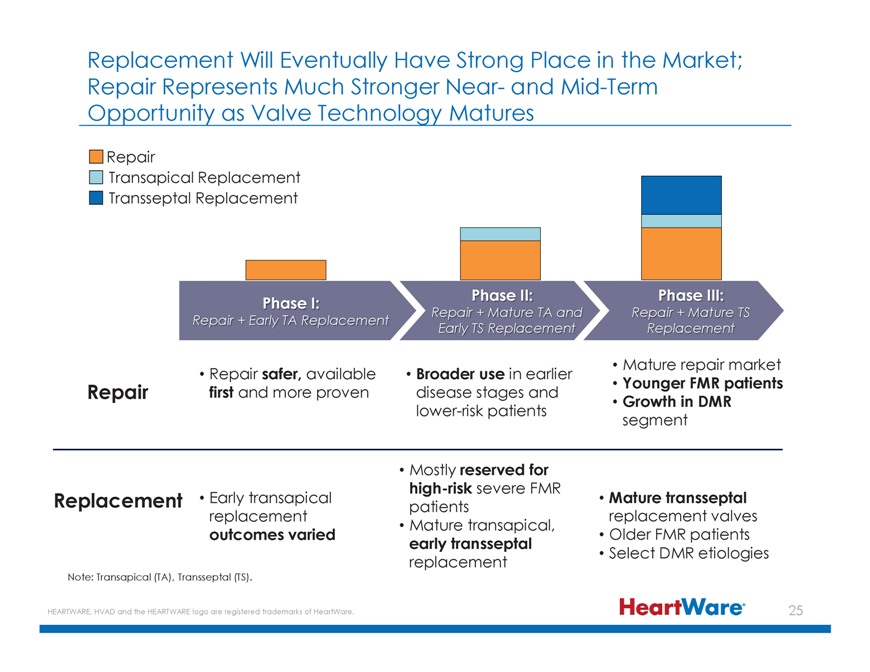
Replacement Will Eventually Have Strong Place in the Market; Repair Represents Much Stronger Near- and Mid-Term Opportunity as Valve Technology Matures Repair Transapical Replacement
Transseptal Replacement Replacement Phase I: Repair + Early TA Replacement R nd Repair + Mature TS Early TS Replacement Replacement Mature repair market Repair first Repair and safer, more available proven disease Broader stages use in earlier and
Younger FMR patients Growth in DMR lower-risk patients segment Mostly reserved for high-risk severe FMR Replacement Early transapical patients Mature transseptal replacement replacement valves Mature transapical, outcomes varied Older FMR patients
early transseptal replacement Select DMR etiologies Note: Transapical (TA), Transseptal (TS). HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
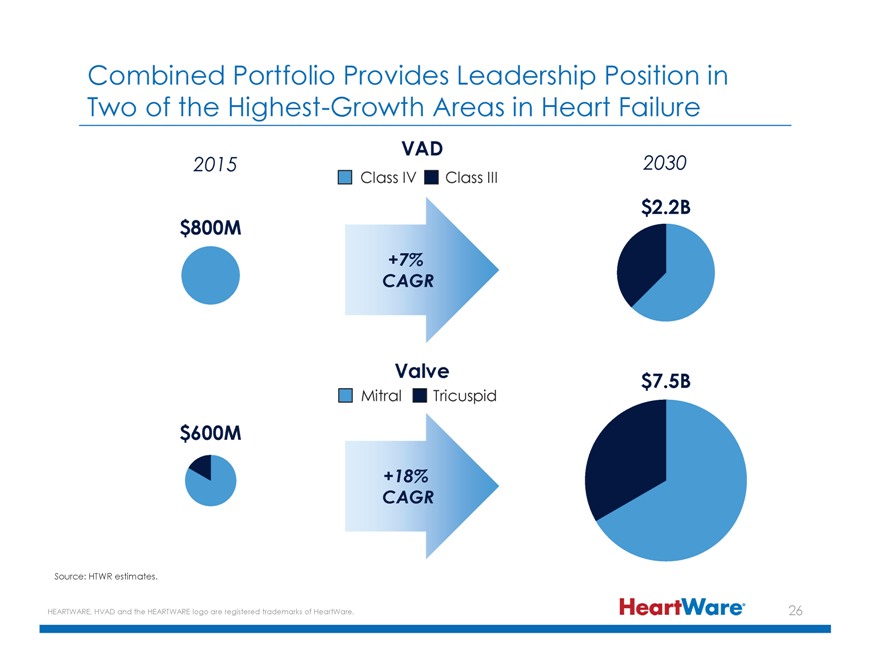
Combined Portfolio Provides Leadership Position in Two of the Highest-Growth Areas in Heart Failure VAD 2015 2030 Class IV Class III $2.2B $800M +7% CAGR Valve $7.5B Mitral Tricuspid $600M
+18% CAGR Source: HTWR estimates. HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.

Last Week in Boston: Analyst and Investor Meeting Led by Renowned VAD and Valve Experts Michael Mack, M.D., FACC Prof. Francesco Maisano, M.D., FESC Director of Cardiovascular Surgery,
Chair of Cardiovascular Surgery, Baylor Scott & White Health University Hospital Zurich Mitral and Tricuspid Regurgitation Valtech Portfolio Overview Overview: The Need, Anatomy, Repair and Replacement Treatment Today and Challenges Prof.
Karl-Heinz Kuck, M.D., Ph.D. Paul Grayburn, M.D., FACC Head of Cardiology, Director of Cardiology Research, St. Georg Hospital Hamburg Baylor Heart and Vascular Institute Cardioband® Deep Dive Cardioband® Deeper Dive: Core Lab Insights Paul
Jansz, BMed, FRACS, Ph.D. Steven Boyce, M.D. Deputy Director of Heart & Lung Director of the Advanced Heart Failure Transplant Program, Surgical Director Program, MedStar Washington Hospital MCS, St. Vincent’s Hospital MVAD®
Overview and The HeartWare-Valtech Combination: User Experience The Physician’s View HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.

For more information and to view the Investor & Analyst Meeting video webcast replay, please visit HeartWare.com HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks
of HeartWare.

Priorities for the Months Ahead… Advance discussions with shareholders regarding proposed Valtech acquisition; facilitate diligence process Complete MVAD® review and implement any
appropriate actions identified Resume enrollment in CE Mark clinical trial of MVAD System Pursue initiation of MVAD System clinical trials in U.S. and Canada Complete Warning Letter remediation process Complete follow-up for ENDURANCE2 DT trial
Secure approval in U.S. for Lavare Cycle Complete enrollment in HVAD® LATERAL thoracotomy IDE study Advance CircuLite® System toward clinic HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.

Thank You HEARTWARE, HVAD and the HEARTWARE logo are registered trademarks of HeartWare.
Heartware International, Inc. (MM) (NASDAQ:HTWR)
Historical Stock Chart
From Mar 2024 to Apr 2024
Heartware International, Inc. (MM) (NASDAQ:HTWR)
Historical Stock Chart
From Apr 2023 to Apr 2024