CEL-SCI to Present at Biotech Showcase 2015
January 09 2015 - 8:30AM
Business Wire
CEL-SCI Corporation (NYSE MKT: CVM), a late-stage
oncology company, announced today that Geert Kersten, Chief
Executive Officer, will be presenting at the Biotech Showcase™ 2015
Conference being held in San Francisco, CA.
Details of CEL-SCI’s presentation are as follows:
Event: Biotech
Showcase 2015 Conference Date: Monday, January 12, 2015
Time: 11:30 am Pacific Time
Location: Parc 55 Wyndham
San Francisco Union Square Hotel
A live audio webcast of the presentation and replay will be
available under the investor relations section of CEL-SCI's website
at www.cel-sci.com. The replay of the presentation will be
available approximately 2 hours after the presentation and is
accessible for 3 months following the event.
About Biotech Showcase 2015
Biotech Showcase 2015 is an investor and partnering conference
devoted to providing private and public biotechnology and life
sciences companies an opportunity to present to, and meet with,
investors and pharmaceutical executives during the course of one of
the industry’s largest annual healthcare investor conferences. Now
in its seventh year, Biotech Showcase is expected to attract
upwards of 1,500 attendees.
About CEL-SCI Corporation
CEL-SCI’s work is focused on finding the best way to activate
the immune system to fight cancer and infectious diseases. Its lead
investigational therapy Multikine* (Leukocyte Interleukin,
Injection) is currently being studied in a pivotal Phase III
clinical trial against head and neck cancer. If the study endpoint,
which is a 10% improvement in overall survival of the subjects
treated with Multikine treatment regimen as compared to subjects
treated with current standard of care only is satisfied, the study
results will be used to support applications which will be
submitted to regulatory agencies in order to receive from these
agencies commercial marketing approvals for Multikine in major
markets around the world. Additional clinical indications for
Multikine which are being investigated include cervical dysplasia
in HIV/HPV co-infected women, and the treatment of peri-anal warts
in HIV/HPV co-infected men and women. A Phase I trial of the former
indication has been completed at the University of Maryland. The
latter indication is now in a Phase I trial in conjunction with the
U.S. Navy under a CRADA (Cooperative Research and Development
Agreement).
CEL-SCI is also developing its LEAPS technology for the
treatment of pandemic influenza and as a potential therapeutic
vaccine against rheumatoid arthritis. The Company has recently
received a Phase I SBIR Grant from the National Institutes of
Health to develop LEAPS as a potential treatment for RA with
researchers from Rush University Medical Center in Chicago,
Illinois. The Company has operations in Vienna, Virginia, and
in/near Baltimore, Maryland.
For more information, please visit www.cel-sci.com.
* Multikine is the trademark that CEL-SCI has registered for
this investigational therapy, and this proprietary name is subject
to FDA review in connection with our future anticipated regulatory
submission for approval. Multikine has not been licensed or
approved for sale, barter or exchange by the FDA or any other
regulatory agency. Similarly, its safety or efficacy has not been
established for any use. Moreover, no definitive conclusions can be
drawn from the early-phase, clinical-trials data involving the
investigational therapy Multikine (Leukocyte Interleukin,
Injection). Further research is required, and early-phase clinical
trial results must be confirmed in the well-controlled, Phase III
clinical trial of this investigational therapy that is currently in
progress.
When used in this report, the words "intends," "believes,"
"anticipated", “plans” and "expects" and similar expressions are
intended to identify forward-looking statements. Such statements
are subject to risks and uncertainties which could cause actual
results to differ materially from those projected. Factors that
could cause or contribute to such differences include, an inability
to duplicate the clinical results demonstrated in clinical studies,
timely development of any potential products that can be shown to
be safe and effective, receiving necessary regulatory approvals,
difficulties in manufacturing any of the Company's potential
products, inability to raise the necessary capital and the risk
factors set forth from time to time in CEL-SCI Corporation's SEC
filings, including but not limited to its report on Form 10- K for
the year ended September 30, 2014. The Company undertakes no
obligation to publicly release the result of any revision to these
forward-looking statements which may be made to reflect the events
or circumstances after the date hereof or to reflect the occurrence
of unanticipated events.
CEL-SCI CorporationGavin de Windt, 703-506-9460
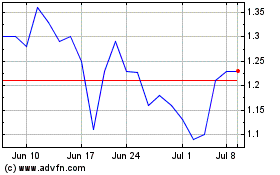
Cel Sci (AMEX:CVM)
Historical Stock Chart
From Mar 2024 to Apr 2024
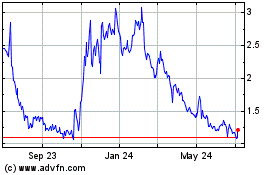
Cel Sci (AMEX:CVM)
Historical Stock Chart
From Apr 2023 to Apr 2024